Sex and APOE Genotype Alter the Basal and Induced Inflammatory States of Primary Microglia from APOE Targeted Replacement Mice
- PMID: 36077227
- PMCID: PMC9456163
- DOI: 10.3390/ijms23179829
Sex and APOE Genotype Alter the Basal and Induced Inflammatory States of Primary Microglia from APOE Targeted Replacement Mice
Abstract
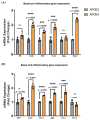
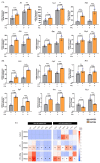
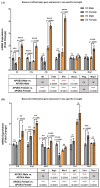
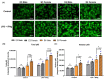
The sex and APOE4 genotype are significant risk factors for Alzheimer’s disease (AD); however, the mechanism(s) responsible for this interaction are still a matter of debate. Here, we assess the responses of mixed-sex and sex-specific APOE3 and APOE4 primary microglia (PMG) to lipopolysaccharide and interferon-gamma. In our investigation, inflammatory cytokine profiles were assessed by qPCR and multiplex ELISA assays. Mixed-sex APOE4 PMG exhibited higher basal mRNA expression and secreted levels of TNFa and IL1b. In sex-specific cultures, basal expression and secreted levels of IL1b, TNFa, IL6, and NOS2 were 2−3 fold higher in APOE4 female PMG compared to APOE4 males, with both higher than APOE3 cells. Following an inflammatory stimulus, the expression of pro-inflammatory cytokines and the secreted cytokine level were upregulated in the order E4 female > E4 male > E3 female > E3 male in sex-specific cultures. These data indicate that the APOE4 genotype and female sex together contribute to a greater inflammatory response in PMG isolated from targeted replacement humanized APOE mice. These data are consistent with clinical data and indicate that sex-specific PMG may provide a platform for exploring mechanisms of genotype and sex differences in AD related to neuroinflammation and neurodegeneration.
Keywords: APOE; Alzheimer’s disease; cytokine; microglia; neuroinflammation; sex.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




Similar articles
-
A small-molecule TLR4 antagonist reduced neuroinflammation in female E4FAD mice.Alzheimers Res Ther. 2023 Oct 19;15(1):181. doi: 10.1186/s13195-023-01330-6. Alzheimers Res Ther. 2023. PMID: 37858252 Free PMC article.
-
Sex and APOE Genotype Alter the Basal and Induced Inflammatory States of Primary Astrocytes from Humanized Targeted Replacement Mice.ASN Neuro. 2023 Jan-Dec;15:17590914221144549. doi: 10.1177/17590914221144549. ASN Neuro. 2023. PMID: 36604975 Free PMC article.
-
APOE genotype-specific differences in the innate immune response.Neurobiol Aging. 2009 Sep;30(9):1350-60. doi: 10.1016/j.neurobiolaging.2007.11.014. Epub 2007 Dec 21. Neurobiol Aging. 2009. PMID: 18155324 Free PMC article.
-
Apolipoprotein E and oxidative stress in brain with relevance to Alzheimer's disease.Neurobiol Dis. 2020 May;138:104795. doi: 10.1016/j.nbd.2020.104795. Epub 2020 Feb 6. Neurobiol Dis. 2020. PMID: 32036033 Free PMC article. Review.
-
Implications of apolipoprotein E genotype on inflammation and vitamin E status.Mol Nutr Food Res. 2010 May;54(5):623-30. doi: 10.1002/mnfr.200900398. Mol Nutr Food Res. 2010. PMID: 20183830 Review.
Cited by
-
Roles of ApoE4 on the Pathogenesis in Alzheimer's Disease and the Potential Therapeutic Approaches.Cell Mol Neurobiol. 2023 Oct;43(7):3115-3136. doi: 10.1007/s10571-023-01365-1. Epub 2023 May 25. Cell Mol Neurobiol. 2023. PMID: 37227619 Free PMC article. Review.
-
APOE4 reshapes the lipid droplet proteome and modulates microglial inflammatory responses.Neurobiol Dis. 2025 Aug;212:106983. doi: 10.1016/j.nbd.2025.106983. Epub 2025 May 30. Neurobiol Dis. 2025. PMID: 40451545 Free PMC article.
-
Senescent Microglia Represent a Subset of Disease-Associated Microglia in P301S Mice.J Alzheimers Dis. 2023;95(2):493-507. doi: 10.3233/JAD-230109. J Alzheimers Dis. 2023. PMID: 37545233 Free PMC article.
-
A small-molecule TLR4 antagonist reduced neuroinflammation in female E4FAD mice.Alzheimers Res Ther. 2023 Oct 19;15(1):181. doi: 10.1186/s13195-023-01330-6. Alzheimers Res Ther. 2023. PMID: 37858252 Free PMC article.
-
The Role of Phytochemicals and Gut Microbiome in Atherosclerosis in Preclinical Mouse Models.Nutrients. 2023 Feb 28;15(5):1212. doi: 10.3390/nu15051212. Nutrients. 2023. PMID: 36904211 Free PMC article. Review.
References
-
- Jones L.P.A., Holmans M.L.P., Hamshere D.M.L., Harold V.D., Moskvina D.V., Ivanov A.D., Pocklington R.A., Abraham P.R., Hollingworth R.P., Sims R., et al. Genetic Evidence Implicates the Immune System and Cholesterol Metabolism in the Aetiology of Alzheimer’s Disease. PLoS ONE. 2010;5:e13950. doi: 10.1371/journal.pone.0013950. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

