Sensitivity of the Transport of Plastic Nanoparticles to Typical Phosphates Associated with Ionic Strength and Solution pH
- PMID: 36077260
- PMCID: PMC9455956
- DOI: 10.3390/ijms23179860
Sensitivity of the Transport of Plastic Nanoparticles to Typical Phosphates Associated with Ionic Strength and Solution pH
Abstract
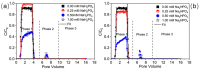
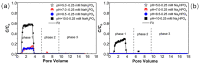
The influence of phosphates on the transport of plastic particles in porous media is environmentally relevant due to their ubiquitous coexistence in the subsurface environment. This study investigated the transport of plastic nanoparticles (PNPs) via column experiments, paired with Derjaguin-Landau-Verwey-Overbeek calculations and numerical simulations. The trends of PNP transport vary with increasing concentrations of NaH2PO4 and Na2HPO4 due to the coupled effects of increased electrostatic repulsion, the competition for retention sites, and the compression of the double layer. Higher pH tends to increase PNP transport due to the enhanced deprotonation of surfaces. The release of retained PNPs under reduced IS and increased pH is limited because most of the PNPs were irreversibly captured in deep primary minima. The presence of physicochemical heterogeneities on solid surfaces can reduce PNP transport and increase the sensitivity of the transport to IS. Furthermore, variations in the hydrogen bonding when the two phosphates act as proton donors will result in different influences on PNP transport at the same IS. This study highlights the sensitivity of PNP transport to phosphates associated with the solution chemistries (e.g., IS and pH) and is helpful for better understanding the fate of PNPs and other colloidal contaminants in the subsurface environment.
Keywords: phosphates; plastic nanoparticles; release; retention; solution chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

