α2δ-4 and Cachd1 Proteins Are Regulators of Presynaptic Functions
- PMID: 36077281
- PMCID: PMC9456004
- DOI: 10.3390/ijms23179885
α2δ-4 and Cachd1 Proteins Are Regulators of Presynaptic Functions
Abstract
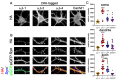
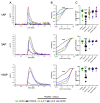
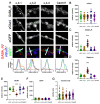
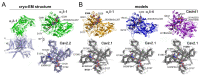
The α2δ auxiliary subunits of voltage-gated calcium channels (VGCC) were traditionally regarded as modulators of biophysical channel properties. In recent years, channel-independent functions of these subunits, such as involvement in synapse formation, have been identified. In the central nervous system, α2δ isoforms 1, 2, and 3 are strongly expressed, regulating glutamatergic synapse formation by a presynaptic mechanism. Although the α2δ-4 isoform is predominantly found in the retina with very little expression in the brain, it was recently linked to brain functions. In contrast, Cachd1, a novel α2δ-like protein, shows strong expression in brain, but its function in neurons is not yet known. Therefore, we aimed to investigate the presynaptic functions of α2δ-4 and Cachd1 by expressing individual proteins in cultured hippocampal neurons. Both α2δ-4 and Cachd1 are expressed in the presynaptic membrane and could rescue a severe synaptic defect present in triple knockout/knockdown neurons that lacked the α2δ-1-3 isoforms (α2δ TKO/KD). This observation suggests that presynaptic localization and the regulation of synapse formation in glutamatergic neurons is a general feature of α2δ proteins. In contrast to this redundant presynaptic function, α2δ-4 and Cachd1 differentially regulate the abundance of presynaptic calcium channels and the amplitude of presynaptic calcium transients. These functional differences may be caused by subtle isoform-specific differences in α1-α2δ protein-protein interactions, as revealed by structural homology modelling. Taken together, our study identifies both α2δ-4 and Cachd1 as presynaptic regulators of synapse formation, differentiation, and calcium channel functions that can at least partially compensate for the loss of α2δ-1-3. Moreover, we show that regulating glutamatergic synapse formation and differentiation is a critical and surprisingly redundant function of α2δ and Cachd1.
Keywords: Cachd1; presynaptic calcium imaging; synapse formation; synaptic differentiation; voltage-gated calcium channels; α2δ subunits.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Calderón-Rivera A., Andrade A., Hernández-Hernández O., González-Ramírez R., Sandoval A., Rivera M., Gomora J.C., Felix R. Identification of a Disulfide Bridge Essential for Structure and Function of the Voltage-Gated Ca2+ Channel α2δ-1 Auxiliary Subunit. Cell Calcium. 2012;51:22–30. doi: 10.1016/j.ceca.2011.10.002. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

