NAD/NAMPT and mTOR Pathways in Melanoma: Drivers of Drug Resistance and Prospective Therapeutic Targets
- PMID: 36077374
- PMCID: PMC9456568
- DOI: 10.3390/ijms23179985
NAD/NAMPT and mTOR Pathways in Melanoma: Drivers of Drug Resistance and Prospective Therapeutic Targets
Abstract
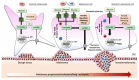
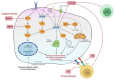
Malignant melanoma represents the most fatal skin cancer due to its aggressive behavior and high metastatic potential. The introduction of BRAF/MEK inhibitors and immune-checkpoint inhibitors (ICIs) in the clinic has dramatically improved patient survival over the last decade. However, many patients either display primary (i.e., innate) or develop secondary (i.e., acquired) resistance to systemic treatments. Therapeutic resistance relies on the rewiring of multiple processes, including cancer metabolism, epigenetics, gene expression, and interactions with the tumor microenvironment that are only partially understood. Therefore, reliable biomarkers of resistance or response, capable of facilitating the choice of the best treatment option for each patient, are currently missing. Recently, activation of nicotinamide adenine dinucleotide (NAD) metabolism and, in particular, of its rate-limiting enzyme nicotinamide phosphoribosyltransferase (NAMPT) have been identified as key drivers of targeted therapy resistance and melanoma progression. Another major player in this context is the mammalian target of rapamycin (mTOR) pathway, which plays key roles in the regulation of melanoma cell anabolic functions and energy metabolism at the switch between sensitivity and resistance to targeted therapy. In this review, we summarize known resistance mechanisms to ICIs and targeted therapy, focusing on metabolic adaptation as one main mechanism of drug resistance. In particular, we highlight the roles of NAD/NAMPT and mTOR signaling axes in this context and overview data in support of their inhibition as a promising strategy to overcome treatment resistance.
Keywords: NAMPT; cancer therapy; drug resistance; mTOR; melanoma; metabolic reprogramming; signaling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022;12:31–46. doi: 10.1158/2159-8290.CD-21-1059. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

