Nephritis-Associated Plasmin Receptor (NAPlr): An Essential Inducer of C3-Dominant Glomerular Injury and a Potential Key Diagnostic Biomarker of Infection-Related Glomerulonephritis (IRGN)
- PMID: 36077377
- PMCID: PMC9456382
- DOI: 10.3390/ijms23179974
Nephritis-Associated Plasmin Receptor (NAPlr): An Essential Inducer of C3-Dominant Glomerular Injury and a Potential Key Diagnostic Biomarker of Infection-Related Glomerulonephritis (IRGN)
Abstract
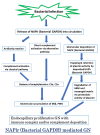
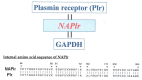
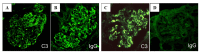
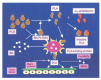
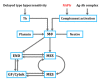
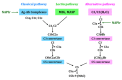
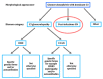
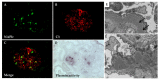
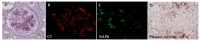
Nephritis-associated plasmin receptor (NAPlr) was originally isolated from the cytoplasmic fraction of group A Streptococci, and was found to be the same molecule as streptococcal glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and plasmin receptor (Plr) on the basis of nucleotide and amino acid sequence homology. Its main functions include GAPDH activity, plasmin-binding capacity, and direct activation of the complement alternative pathway (A-P). Plasmin trapped by deposited NAPlr triggers the degradation of extracellular matrix proteins, such as glomerular basement membranes and mesangial matrix, and the accumulation of macrophages and neutrophils, leading to the induction of plasmin-related endocapillary glomerular inflammation. Deposited NAPlr at glomerular endocapillary site directly activates the complement A-P, and the endocapillary release of complement-related anaphylatoxins, C3a and C5a, amplify the in situ endocapillary glomerular inflammation. Subsequently, circulating and in situ-formed immune complexes participate in the glomerular injury resulting in NAPlr-mediated glomerulonephritis. The disease framework of infection-related glomerulonephritis (IRGN) has been further expanded. GAPDH of various bacteria other than Streptococci have been found to react with anti-NAPlr antibodies and to possess plasmin-binding activities, allowing glomerular NAPlr and plasmin activity to be utilized as key biomarkers of IRGN.
Keywords: C3-dominant glomerular injury; NAPlr (Bacterial GAPDH) mediated glomerulonephritis; immune complex-dominant glomerular injury; infection-related glomerulonephritis (IRGN); nephritis-associated plasmin receptor (NAPlr); poststreptococcal acute glomerulonephritis (PSAGN).
Conflict of interest statement
The authors declare no conflict of interest.
Figures


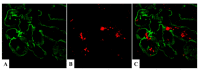




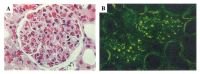



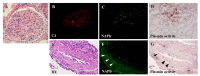
References
-
- Yoshizawa N., Yamakami K., Fujino M., Oda T., Tamura K., Matsumoto K., Sugisaki T., Boyle M.D. Nephritis-associated plasmin receptor and acute poststreptococcal glomerulonephritis: Characterization of the antigen and associated immune response. J. Am. Soc. Nephrol. 2004;15:1785–1793. doi: 10.1097/01.ASN.0000130624.94920.6B. - DOI - PubMed
-
- Couser W.G., Johnson R.J. Post-infecitous glomerulonephritis. In: Neilson E.G., Couser W.G., editors. Immunologic Renal Diseases. Lippincott-Raven; Philadelphia, PA, USA: 1997. pp. 915–943.
-
- Nadasdy T., Silva F.G. Acute postinfectious glomerulonephritis and glomerulonephritis complicating persistent bacterial infection. In: Jennette J.C., Olson J.L., Schwartz M.M., Silva F.G., editors. Heptinstall’s Pathology of the Kidney. 6th ed. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2007. pp. 321–396.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

