Effect of Titanium and Zirconia Nanoparticles on Human Gingival Mesenchymal Stromal Cells
- PMID: 36077419
- PMCID: PMC9456558
- DOI: 10.3390/ijms231710022
Effect of Titanium and Zirconia Nanoparticles on Human Gingival Mesenchymal Stromal Cells
Abstract
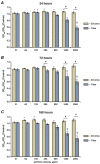
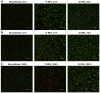
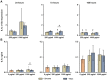
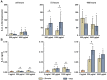
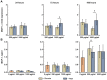
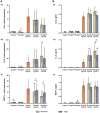
Nano- and microparticles are currently being discussed as potential risk factors for peri-implant disease. In the present study, we compared the responses of human gingival mesenchymal stromal cells (hG-MSCs) on titanium and zirconia nanoparticles (<100 nm) in the absence and presence of Porphyromonas gingivalis lipopolysaccharide (LPS). The primary hG-MSCs were treated with titanium and zirconia nanoparticles in concentrations up to 2.000 µg/mL for 24 h, 72 h, and 168 h. Additionally, the cells were treated with different nanoparticles (25−100 µg/mL) in the presence of P. gingivalis LPS for 24 h. The cell proliferation and viability assay and live−dead and focal adhesion stainings were performed, and the expression levels of interleukin (IL)-6, IL-8, and monocyte chemoattractant protein (MCP)-1 were measured. The cell proliferation and viability were inhibited by the titanium (>1000 µg/mL) but not the zirconia nanoparticles, which was accompanied by enhanced apoptosis. Both types of nanoparticles (>25 µg/mL) induced the significant expression of IL-8 in gingival MSCs, and a slightly higher effect was observed for titanium nanoparticles. Both nanoparticles substantially enhanced the P. gingivalis LPS-induced IL-8 production; a higher effect was observed for zirconia nanoparticles. The production of inflammatory mediators by hG-MSCs is affected by the nanoparticles. This effect depends on the nanoparticle material and the presence of inflammatory stimuli.
Keywords: dental implants; human gingival mesenchymal stromal cells; nanoparticles; titanium; zirconia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

