Albumin/Thiacalix[4]arene Nanoparticles as Potential Therapeutic Systems: Role of the Macrocycle for Stabilization of Monomeric Protein and Self-Assembly with Ciprofloxacin
- PMID: 36077448
- PMCID: PMC9455997
- DOI: 10.3390/ijms231710040
Albumin/Thiacalix[4]arene Nanoparticles as Potential Therapeutic Systems: Role of the Macrocycle for Stabilization of Monomeric Protein and Self-Assembly with Ciprofloxacin
Abstract
The therapeutic application of serum albumin is determined by the relative content of the monomeric form compared to dimers, tetramers, hexamers, etc. In this paper, we propose and develop an approach to synthesize the cone stereoisomer of p-tert-butylthiacalix[4]arene with sulfobetaine fragments stabilization of monomeric bovine serum albumin and preventing aggregation. Spectral methods (UV-vis, CD, fluorescent spectroscopy, and dynamic light scattering) established the influence of the synthesized compounds on the content of monomeric and aggregated forms of BSA even without the formation of stable thiacalixarene/protein associates. The effect of thiacalixarenes on the efficiency of protein binding with the antibiotic ciprofloxacin was shown by fluorescence spectroscopy. The binding constant increases in the presence of the macrocycles, likely due to the stabilization of monomeric forms of BSA. Our study clearly shows the potential of this macrocycle design as a platform for the development of the fundamentally new approaches for preventing aggregation.
Keywords: bovine serum albumin; ciprofloxacin; self-assembly; sulfobetaines; thiacalixarene.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
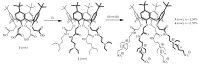






References
-
- Yurina E.S., Gubarev Y.A., Lebedeva N.S. A study of protein aggregation activators in molecular complexes of cationic porphyrins and chlorin with BSA. J. Mol. Liq. 2021;338:116632. doi: 10.1016/j.molliq.2021.116632. - DOI
-
- Holm N.K., Jespersen S.K., Thomassen L.V., Wolff T.Y., Sehgal P., Thomsen L.A., Christiansen G., Andersen C.B., Knudsen A.D., Otzen D.E. Aggregation and fibrillation of bovine serum albumin. Biochim. Biophys. Acta-Proteins Proteom. 2007;1774:1128–1138. doi: 10.1016/j.bbapap.2007.06.008. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

