eif4ebp3l-A New Affector of Zebrafish Angiogenesis and Heart Regeneration?
- PMID: 36077472
- PMCID: PMC9456460
- DOI: 10.3390/ijms231710075
eif4ebp3l-A New Affector of Zebrafish Angiogenesis and Heart Regeneration?
Abstract
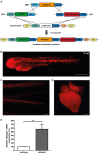
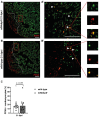
The eukaryotic initiation factor 4E binding protein (4E-BP) family is involved in translational control of cell proliferation and pro-angiogenic factors. The zebrafish eukaryotic initiation factor 4E binding protein 3 like (eif4ebp3l) is a member of the 4E-BPs and responsible for activity-dependent myofibrillogenesis, but whether it affects cardiomyocyte (CM) proliferation or heart regeneration is unclear. We examined eif4ebp3l during zebrafish vascular development and heart regeneration post cryoinjury in adult zebrafish. Using morpholino injections we induced silencing of eif4ebp3l in zebrafish embryos, which led to increased angiogenesis at 94 h post fertilization (hpf). For investigation of eif4ebp3l in cardiac regeneration, zebrafish hearts were subjected to cryoinjury. Regenerating hearts were analyzed at different time points post-cryoinjury for expression of eif4ebp3l by in situ hybridization and showed strongly decreased eif4ebp3l expression in the injured area. We established a transgenic zebrafish strain, which overexpressed eif4ebp3l under the control of a heat-shock dependent promotor. Overexpression of eif4ebp3l during zebrafish heart regeneration caused only macroscopically a reduced amount of fibrin at the site of injury. Overall, these findings demonstrate that silencing of eif4ebp3l has pro-angiogenic properties in zebrafish vascular development and when eif4ebp3l is overexpressed, fibrin deposition tends to be altered in zebrafish cardiac regeneration after cryoinjury.
Keywords: 4E-BPs; heart; myocardial infarction; regeneration; zebrafish.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
-
- Khan M.A., Hashim M.J., Mustafa H., Baniyas M.Y., Al Suwaidi S., AlKatheeri R., Alblooshi F.M.K., Almatrooshi M., Alzaabi M.E.H., Al Darmaki R.S., et al. Global Epidemiology of Ischemic Heart Disease: Results from the Global Burden of Disease Study. Cureus. 2020;12:e9349. doi: 10.7759/cureus.9349. - DOI - PMC - PubMed
-
- Gerber Y., Weston S.A., Enriquez-Sarano M., Berardi C., Chamberlain A.M., Manemann S.M., Jiang R., Dunlay S.M., Roger V.L. Mortality Associated with Heart Failure after Myocardial Infarction: A Contemporary Community Perspective. Circ. Heart Fail. 2016;9:e002460. doi: 10.1161/CIRCHEARTFAILURE.115.002460. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

