TNF-α Plus IL-1β Induces Opposite Regulation of Cx43 Hemichannels and Gap Junctions in Mesangial Cells through a RhoA/ROCK-Dependent Pathway
- PMID: 36077498
- PMCID: PMC9456118
- DOI: 10.3390/ijms231710097
TNF-α Plus IL-1β Induces Opposite Regulation of Cx43 Hemichannels and Gap Junctions in Mesangial Cells through a RhoA/ROCK-Dependent Pathway
Abstract
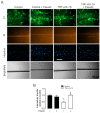
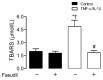
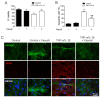
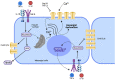
Connexin 43 (Cx43) is expressed in kidney tissue where it forms hemichannels and gap junction channels. However, the possible functional relationship between these membrane channels and their role in damaged renal cells remains unknown. Here, analysis of ethidium uptake and thiobarbituric acid reactive species revealed that treatment with TNF-α plus IL-1β increases Cx43 hemichannel activity and oxidative stress in MES-13 cells (a cell line derived from mesangial cells), and in primary mesangial cells. The latter was also accompanied by a reduction in gap junctional communication, whereas Western blotting assays showed a progressive increase in phosphorylated MYPT (a target of RhoA/ROCK) and Cx43 upon TNF-α/IL-1β treatment. Additionally, inhibition of RhoA/ROCK strongly antagonized the TNF-α/IL-1β-induced activation of Cx43 hemichannels and reduction in gap junctional coupling. We propose that activation of Cx43 hemichannels and inhibition of cell-cell coupling during pro-inflammatory conditions could contribute to oxidative stress and damage of mesangial cells via the RhoA/ROCK pathway.
Keywords: Fasudil; Y-27632; connexin hemichannel; gap junction; inflammatory receptors; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Angiotensin II-Induced Mesangial Cell Damaged Is Preceded by Cell Membrane Permeabilization Due to Upregulation of Non-Selective Channels.Int J Mol Sci. 2018 Mar 23;19(4):957. doi: 10.3390/ijms19040957. Int J Mol Sci. 2018. PMID: 29570626 Free PMC article.
-
Connexin 43 Hemichannel Activity Promoted by Pro-Inflammatory Cytokines and High Glucose Alters Endothelial Cell Function.Front Immunol. 2018 Aug 15;9:1899. doi: 10.3389/fimmu.2018.01899. eCollection 2018. Front Immunol. 2018. PMID: 30158937 Free PMC article.
-
Cx43 hemichannels and gap junction channels in astrocytes are regulated oppositely by proinflammatory cytokines released from activated microglia.J Neurosci. 2007 Dec 12;27(50):13781-92. doi: 10.1523/JNEUROSCI.2042-07.2007. J Neurosci. 2007. PMID: 18077690 Free PMC article.
-
Peptides and peptide-derived molecules targeting the intracellular domains of Cx43: gap junctions versus hemichannels.Neuropharmacology. 2013 Dec;75:491-505. doi: 10.1016/j.neuropharm.2013.04.050. Epub 2013 May 7. Neuropharmacology. 2013. PMID: 23664811 Review.
-
Connexin 43 and ischemic preconditioning.Cardiovasc Res. 2004 May 1;62(2):335-44. doi: 10.1016/j.cardiores.2003.12.017. Cardiovasc Res. 2004. PMID: 15094353 Review.
Cited by
-
Activation of Pannexin-1 channels causes cell dysfunction and damage in mesangial cells derived from angiotensin II-exposed mice.Front Cell Dev Biol. 2024 Apr 9;12:1387234. doi: 10.3389/fcell.2024.1387234. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38660621 Free PMC article.
-
Hygrothermal stress increases malignant arrhythmias susceptibility by inhibiting the LKB1-AMPK-Cx43 pathway.Sci Rep. 2024 Feb 29;14(1):5010. doi: 10.1038/s41598-024-55804-0. Sci Rep. 2024. PMID: 38424223 Free PMC article.
References
-
- Yang Z.-J., Wang H.-R., Wang Y.-I., Zhai Z.-H., Wang L.-W., Li L., Zhang C., Tang L. Myricetin Attenuated Diabetes-Associated Kidney Injuries and Dysfunction via Regulating Nuclear Factor (Erythroid Derived 2)-Like 2 and Nuclear Factor-κB Signaling. Front. Pharmacol. 2019;10:647. doi: 10.3389/fphar.2019.00647. - DOI - PMC - PubMed
-
- Zhao J.-H. Renal Fibrosis: Mechanisms and Therapies. Advances in Experimental Medicine and Biology. Springer; Singapore: 2019. Mesangial Cells and Renal Fibrosis; pp. 165–194. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

