Factors and Pathways Modulating Endothelial Cell Senescence in Vascular Aging
- PMID: 36077539
- PMCID: PMC9456027
- DOI: 10.3390/ijms231710135
Factors and Pathways Modulating Endothelial Cell Senescence in Vascular Aging
Abstract
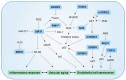
Aging causes a progressive decline in the structure and function of organs. With advancing age, an accumulation of senescent endothelial cells (ECs) contributes to the risk of developing vascular dysfunction and cardiovascular diseases, including hypertension, diabetes, atherosclerosis, and neurodegeneration. Senescent ECs undergo phenotypic changes that alter the pattern of expressed proteins, as well as their morphologies and functions, and have been linked to vascular impairments, such as aortic stiffness, enhanced inflammation, and dysregulated vascular tone. Numerous molecules and pathways, including sirtuins, Klotho, RAAS, IGFBP, NRF2, and mTOR, have been implicated in promoting EC senescence. This review summarizes the molecular players and signaling pathways driving EC senescence and identifies targets with possible therapeutic value in age-related vascular diseases.
Keywords: age-related vascular disease; cellular senescence; endothelial cell; molecular player; putative target; signaling pathway.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Demaria M., Ohtani N., Youssef S.A., Rodier F., Toussaint W., Mitchell J.R., Laberge R.M., Vijg J., Van Steeg H., Dollé M.E., et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell. 2014;31:722–733. doi: 10.1016/j.devcel.2014.11.012. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

