Dapagliflozin Mitigates Doxorubicin-Caused Myocardium Damage by Regulating AKT-Mediated Oxidative Stress, Cardiac Remodeling, and Inflammation
- PMID: 36077544
- PMCID: PMC9456438
- DOI: 10.3390/ijms231710146
Dapagliflozin Mitigates Doxorubicin-Caused Myocardium Damage by Regulating AKT-Mediated Oxidative Stress, Cardiac Remodeling, and Inflammation
Abstract
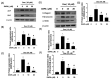
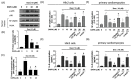
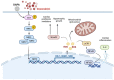
Doxorubicin (Dox) is a commonly used anthracycline chemotherapy with a side effect of cardiotoxicity, which may increase the risk of heart failure for cancer patients. Although various studies have demonstrated the cardioprotective property of dapagliflozin (DAPA), a sodium-glucose cotransporter 2 inhibitor, the detailed mechanism underlying its effect on Dox-induced cardiomyopathy is still limited. In this study, we showed that DAPA induced the activation of AKT/PI3K signaling in cardiac myoblast H9c2 cells following Dox treatment, leading to the upregulation of antioxidant HO-1, NQO1, and SOD, as well as an improved mitochondrial dysfunction via Nrf2. In addition, the reduced oxidative stress resulted in the downregulation of hypertrophy (ANP and BNP) and fibrosis (phospho-Smad3, collagen I, fibronectin, and α-SMA) markers. Furthermore, the inflammatory IL-8 concentration was inhibited after DAPA, possibly through PI3K/AKT/Nrf2/p38/NF-κB signaling. Moreover, our results were validated in vivo, and echocardiography results suggested an improved cardiac function in DAPA-receiving rats. In summary, we demonstrated that the administration of DAPA could mitigate the Dox-elicited cardiotoxicity by reducing oxidative stress, mitochondrial dysfunction, fibrosis, hypertrophy, and inflammation via PI3K/AKT/Nrf2 signaling.
Keywords: Akt; cardiotoxicity; dapagliflozin; doxorubicin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Cardinale D., Colombo A., Bacchiani G., Tedeschi I., Meroni C.A., Veglia F., Civelli M., Lamantia G., Colombo N., Curigliano G., et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131:1981–1988. doi: 10.1161/CIRCULATIONAHA.114.013777. - DOI - PubMed
-
- McMurray J.J.V., Solomon S.D., Inzucchi S.E., Køber L., Kosiborod M.N., Martinez F.A., Ponikowski P., Sabatine M.S., Anand I.S., Bělohlávek J., et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

