Connexins and Glucose Metabolism in Cancer
- PMID: 36077565
- PMCID: PMC9455984
- DOI: 10.3390/ijms231710172
Connexins and Glucose Metabolism in Cancer
Abstract
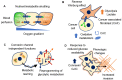
Connexins are a family of transmembrane proteins that regulate diverse cellular functions. Originally characterized for their ability to mediate direct intercellular communication through the formation of highly regulated membrane channels, their functions have been extended to the exchange of molecules with the extracellular environment, and the ability to modulate numerous channel-independent effects on processes such as motility and survival. Notably, connexins have been implicated in cancer biology for their context-dependent roles that can both promote or suppress cancer cell function. Moreover, connexins are able to mediate many aspects of cellular metabolism including the intercellular coupling of nutrients and signaling molecules. During cancer progression, changes to substrate utilization occur to support energy production and biomass accumulation. This results in metabolic plasticity that promotes cell survival and proliferation, and can impact therapeutic resistance. Significant progress has been made in our understanding of connexin and cancer biology, however, delineating the roles these multi-faceted proteins play in metabolic adaptation of cancer cells is just beginning. Glucose represents a major carbon substrate for energy production, nucleotide synthesis, carbohydrate modifications and generation of biosynthetic intermediates. While cancer cells often exhibit a dependence on glycolytic metabolism for survival, cellular reprogramming of metabolic pathways is common when blood perfusion is limited in growing tumors. These metabolic changes drive aggressive phenotypes through the acquisition of functional traits. Connections between glucose metabolism and connexin function in cancer cells and the surrounding stroma are now apparent, however much remains to be discovered regarding these relationships. This review discusses the existing evidence in this area and highlights directions for continued investigation.
Keywords: connexin; gap junction; glucose; metabolism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

