Antenatal Glucocorticoid Administration Promotes Cardiac Structure and Energy Metabolism Maturation in Preterm Fetuses
- PMID: 36077580
- PMCID: PMC9456503
- DOI: 10.3390/ijms231710186
Antenatal Glucocorticoid Administration Promotes Cardiac Structure and Energy Metabolism Maturation in Preterm Fetuses
Abstract
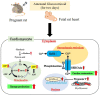
Although the rate of preterm birth has increased in recent decades, a number of preterm infants have escaped death due to improvements in perinatal and neonatal care. Antenatal glucocorticoid (GC) therapy has significantly contributed to progression in lung maturation; however, its potential effects on other organs remain controversial. Furthermore, the effects of antenatal GC therapy on the fetal heart show both pros and cons. Translational research in animal models indicates that constant fetal exposure to antenatal GC administration is sufficient for lung maturation. We have established a premature fetal rat model to investigate immature cardiopulmonary functions in the lungs and heart, including the effects of antenatal GC administration. In this review, we explain the mechanisms of antenatal GC actions on the heart in the fetus compared to those in the neonate. Antenatal GCs may contribute to premature heart maturation by accelerating cardiomyocyte proliferation, angiogenesis, energy production, and sarcoplasmic reticulum function. Additionally, this review specifically focuses on fetal heart growth with antenatal GC administration in experimental animal models. Moreover, knowledge regarding antenatal GC administration in experimental animal models can be coupled with that from developmental biology, with the potential for the generation of functional cells and tissues that could be used for regenerative medical purposes in the future.
Keywords: antenatal glucocorticoid; cardiac growth; fetal heart.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Purisch S.E., Gyamfi-Bannerman C. Epidemiology of preterm birth. Semin. Perinatol. 2017;41:387–391. - PubMed
-
- Platt M.J. Outcomes in preterm infants. Public Health. 2014;128:399–403. - PubMed
-
- Simeoni U., Armengaud J.B., Siddeek B., Tolsa J.F. Perinatal origins of adult disease. Neonatology. 2018;113:393–399. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

