Advantage of Dimethyl Sulfoxide in the Fabrication of Binder-Free Layered Double Hydroxides Electrodes: Impacts of Physical Parameters on the Crystalline Domain and Electrochemical Performance
- PMID: 36077588
- PMCID: PMC9456269
- DOI: 10.3390/ijms231710192
Advantage of Dimethyl Sulfoxide in the Fabrication of Binder-Free Layered Double Hydroxides Electrodes: Impacts of Physical Parameters on the Crystalline Domain and Electrochemical Performance
Abstract
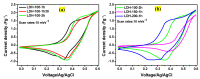
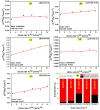
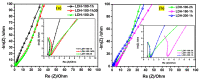
The electrode fabrication stage is a crucial step in the design of supercapacitors. The latter involves the binder generally for adhesive purposes. The binder is electrochemically dormant and has weak interactions, leading to isolating the active material and conductive additive and then compromising the electrochemical performance. Designing binder-free electrodes is a practical way to improve the electrochemical performance of supercapacitors. However, most of the methods developed for the fabrication of binder-free LDH electrodes do not accommodate LDH materials prepared via the co-precipitation or ions exchange routes. Herein, we developed a novel method to fabricate binder-free LDH electrodes which accommodates LDH materials from other synthesis routes. The induced impacts of various physical parameters such as the temperature and time applied during the fabrication process on the crystalline domain and electrochemical performances of all the binder-free LDH electrodes were studied. The electrochemical analysis showed that the electrode prepared at 200 °C-1 h exhibited the best electrochemical performance compared to its counterparts. A specific capacitance of 3050.95 Fg-1 at 10 mVs-1 was achieved by it, while its Rct value was 0.68 Ω. Moreover, it retained 97% of capacitance after 5000 cycles at 120 mVs-1. The XRD and FTIR studies demonstrated that its excellent electrochemical performance was due to its crystalline domain which had held an important amount of water than other electrodes. The as-developed method proved to be reliable and advantageous due to its simplicity and cost-effectiveness.
Keywords: binder-free LDH electrode; dimethyl sulfoxide; layered double hydroxides; supercapacitor.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures







References
-
- Schmidt A., Ramos M.K., Pinto C.S., Pereira A.F., Souza V.H.R., Zarbin A.J.G. Electrode fabrication at liquid interfaces: Towards transparency and flexibility. Electrochem. Commun. 2022;134:107183. doi: 10.1016/j.elecom.2021.107183. - DOI
-
- Shen K., Zhai S., Wang S., Ru Q., Hou X., San Hui K., Nam Hui K., Chen F. Recent Progress in Binder-Free Electrodes Synthesis for Electrochemical Energy Storage Application. Batter. Supercaps. 2021;4:860–880. doi: 10.1002/batt.202000271. - DOI
-
- Gao X., Zhang R., Huang X., Shi Y., Wang C., Gao Y., Han Z. One-step growth of NiCoAl layered double hydroxides microspheres toward high energy density supercapacitors. J. Alloys Compd. 2021;859:157879. doi: 10.1016/j.jallcom.2020.157879. - DOI
-
- Wang X., Li H., Li H., Lin S., Bai J., Dai J., Liang C., Zhu X., Sun Y., Dou S. Heterostructures of Ni-Co-Al layered double hydroxide assembled on V 4 C 3 MXene for high-energy hybrid supercapacitors. J. Mater. Chem. A. 2019;7:2291–2300. doi: 10.1039/C8TA11249E. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

