Multiple Myeloma Therapy: Emerging Trends and Challenges
- PMID: 36077618
- PMCID: PMC9454959
- DOI: 10.3390/cancers14174082
Multiple Myeloma Therapy: Emerging Trends and Challenges
Abstract
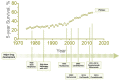
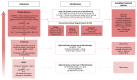
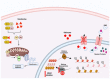
Multiple myeloma (MM) is a complex hematologic malignancy characterized by the uncontrolled proliferation of clonal plasma cells in the bone marrow that secrete large amounts of immunoglobulins and other non-functional proteins. Despite decades of progress and several landmark therapeutic advancements, MM remains incurable in most cases. Standard of care frontline therapies have limited durable efficacy, with the majority of patients eventually relapsing, either early or later. Induced drug resistance via up-modulations of signaling cascades that circumvent the effect of drugs and the emergence of genetically heterogeneous sub-clones are the major causes of the relapsed-refractory state of MM. Cytopenias from cumulative treatment toxicity and disease refractoriness limit therapeutic options, hence creating an urgent need for innovative approaches effective against highly heterogeneous myeloma cell populations. Here, we present a comprehensive overview of the current and future treatment paradigm of MM, and highlight the gaps in therapeutic translations of recent advances in targeted therapy and immunotherapy. We also discuss the therapeutic potential of emerging preclinical research in multiple myeloma.
Keywords: immunotherapy; multiple myeloma; targeted therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Short K.D., Rajkumar S.V., Larson D., Buadi F., Hayman S., Dispenzieri A., Gertz M., Kumar S., Mikhael J., Roy V., et al. Incidence of extramedullary disease in patients with multiple myeloma in the era of novel therapy, and the activity of pomalidomide on extramedullary myeloma. Leukemia. 2011;25:906–908. doi: 10.1038/leu.2011.29. - DOI - PMC - PubMed
-
- Rajkumar S.V., Dimopoulos M.A., Palumbo A., Blade J., Merlini G., Mateos M.V., Kumar S., Hillengass J., Kastritis E., Richardson P., et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538–e548. doi: 10.1016/S1470-2045(14)70442-5. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

