Reversing PD-1 Resistance in B16F10 Cells and Recovering Tumour Immunity Using a COX2 Inhibitor
- PMID: 36077671
- PMCID: PMC9455073
- DOI: 10.3390/cancers14174134
Reversing PD-1 Resistance in B16F10 Cells and Recovering Tumour Immunity Using a COX2 Inhibitor
Abstract
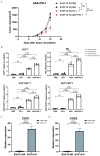
Immunotherapy is an effective method for tumour treatment. Anti-programmed cell death protein 1 (PD-1) and anti-programmed death-ligand 1 (PD-L1) monoclonal antibodies play a significant role in immunotherapy of most tumours; however, some patients develop drug resistance to PD-1/PD-L1 therapy. Cyclooxygenase-2 (COX2) is expressed in various solid tumours, and prostaglandin E2 (PGE2) drives the development of malignant tumours. We developed a drug-resistant B16F10 (B16F10-R) tumour mouse model through four rounds of selection in vivo. Subsequently, we investigated changes in PD-L1 expression and lymphocyte infiltration in B16F10-NR and B16F10-R tumours. Additionally, we explored the role of COX2 in acquired resistance to pembrolizumab, an anti-PD-1 treatment. Immune cell infiltration was significantly decreased in resistant tumours compared to B16F10-NR tumours; however, ptgs2 gene expression was significantly elevated in resistant tumours. Aspirin or celecoxib combined with pembrolizumab can effectively reverse tumour drug resistance. In addition, ptgs2 knockout or the use of the EP4 inhibitor E7046 abrogated drug resistance to anti-PD-1 treatment in B16F10-R tumour cells. Our study showed that inhibition of the COX2/PGE2/EP4 axis could increase the number of immune cells infiltrating the tumour microenvironment and recover drug-resistant tumour sensitivity to pembrolizumab. Thus, we highlight COX2 inhibition as a promising therapeutic target for drug-resistant tumours for future consideration.
Keywords: cyclooxygenase-2; immunosuppression; programmed death-ligand 1; tumour resistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

