Demonstrating Tumor Vascular Disrupting Activity of the Small-Molecule Dihydronaphthalene Tubulin-Binding Agent OXi6196 as a Potential Therapeutic for Cancer Treatment
- PMID: 36077745
- PMCID: PMC9454770
- DOI: 10.3390/cancers14174208
Demonstrating Tumor Vascular Disrupting Activity of the Small-Molecule Dihydronaphthalene Tubulin-Binding Agent OXi6196 as a Potential Therapeutic for Cancer Treatment
Abstract
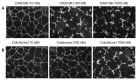
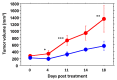
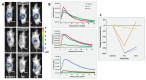
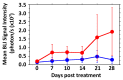
The vascular disrupting activity of a promising tubulin-binding agent (OXi6196) was demonstrated in mice in MDA-MB-231 human breast tumor xenografts growing orthotopically in mammary fat pad and syngeneic RENCA kidney tumors growing orthotopically in the kidney. To enhance water solubility, OXi6196, was derivatized as its corresponding phosphate prodrug salt OXi6197, facilitating effective delivery. OXi6197 is stable in water, but rapidly releases OXi6196 in the presence of alkaline phosphatase. At low nanomolar concentrations OXi6196 caused G2/M cell cycle arrest and apoptosis in MDA-MB-231 breast cancer cells and monolayers of rapidly growing HUVECs underwent concentration-dependent changes in their morphology. Loss of the microtubule structure and increased bundling of filamentous actin into stress fibers followed by cell collapse, rounding and blebbing was observed. OXi6196 (100 nM) disrupted capillary-like endothelial networks pre-established with HUVECs on Matrigel®. When prodrug OXi6197 was administered to mice bearing orthotopic MDA-MB-231-luc tumors, dynamic bioluminescence imaging (BLI) revealed dose-dependent vascular shutdown with >80% signal loss within 2 h at doses ≥30 mg/kg and >90% shutdown after 6 h for doses ≥35 mg/kg, which remained depressed by at least 70% after 24 h. Twice weekly treatment with prodrug OXi6197 (20 mg/kg) caused a significant tumor growth delay, but no overall survival benefit. Similar efficacy was observed for the first time in orthotopic RENCA-luc tumors, which showed massive hemorrhage and necrosis after 24 h. Twice weekly dosing with prodrug OXi6197 (35 mg/kg) caused tumor growth delay in most orthotopic RENCA tumors. Immunohistochemistry revealed extensive necrosis, though with surviving peripheral tissues. These results demonstrate effective vascular disruption at doses comparable to the most effective vascular-disrupting agents (VDAs) suggesting opportunities for further development.
Keywords: bioluminescence imaging (BLI); breast tumors; combretastatin; dihydronaphthalene; kidney tumors; lung tumors; vascular-disrupting agent (VDA).
Conflict of interest statement
The funders had no role in the design of the study; in the collection, analyses or interpretation of the data; in the writing of the manuscript or in the decision to publish the results. K.G.P. holds several patents relevant to the synthesis and use of vascular-disrupting agents.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

