Aberrant Sialylation in Cancer: Therapeutic Opportunities
- PMID: 36077781
- PMCID: PMC9454432
- DOI: 10.3390/cancers14174248
Aberrant Sialylation in Cancer: Therapeutic Opportunities
Abstract
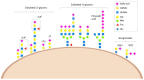
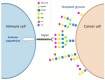
The surface of every eukaryotic cell is coated in a thick layer of glycans that acts as a key interface with the extracellular environment. Cancer cells have a different 'glycan coat' to healthy cells and aberrant glycosylation is a universal feature of cancer cells linked to all of the cancer hallmarks. This means glycans hold huge potential for the development of new diagnostic and therapeutic strategies. One key change in tumour glycosylation is increased sialylation, both on N-glycans and O-glycans, which leads to a dense forest of sialylated structures covering the cell surface. This hypersialylation has far-reaching consequences for cancer cells, and sialylated glycans are fundamental in tumour growth, metastasis, immune evasion and drug resistance. The development of strategies to inhibit aberrant sialylation in cancer represents an important opportunity to develop new therapeutics. Here, I summarise recent advances to target aberrant sialylation in cancer, including the development of sialyltransferase inhibitors and strategies to inhibit Siglecs and Selectins, and discuss opportunities for the future.
Keywords: aberrant sialylation; cancer; glycans; glycosylation; hypersialylation; sialic acid; therapeutics.
Conflict of interest statement
J.M. is a shareholder and Director of GlycoScoreDx Ltd. The funders had no role in the writing of the manuscript or in the decision to publish the results.
Figures

References
-
- Varki A., Kornfeld S. Historical Background and Overview. In: Varki A., Cummings R.D., Esko J.D., Stanley P., Hart G.W., Aebi M., Mohnen D., Kinoshita T., Packer N.H., Prestegard J.H., et al., editors. Essentials of Glycobiology. Cold Spring Harbor; New York, NY, USA: 2022. pp. 1–20. - PubMed
-
- Gagneux P., Hennet T., Varki A. Biological Functions of Glycans. In: Varki A., Cummings R.D., Esko J.D., Stanley P., Hart G.W., Aebi M., Mohnen D., Kinoshita T., Packer N.H., Prestegard J.H., et al., editors. Essentials of Glycobiology. Cold Spring Harbor; New York, NY, USA: 2022. pp. 79–92.
-
- Stanley P., Moremen K.W., Lewis N.E., Taniguchi N., Aebi M. N-Glycans. In: Varki A., Cummings R.D., Esko J.D., Stanley P., Hart G.W., Aebi M., Mohnen D., Kinoshita T., Packer N.H., Prestegard J.H., et al., editors. Essentials of Glycobiology. Cold Spring Harbor; New York, NY, USA: 2022. pp. 103–116.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

