Nucleolin Therapeutic Targeting Decreases Pancreatic Cancer Immunosuppression
- PMID: 36077801
- PMCID: PMC9454580
- DOI: 10.3390/cancers14174265
Nucleolin Therapeutic Targeting Decreases Pancreatic Cancer Immunosuppression
Erratum in
-
Correction: Ponzo et al. Nucleolin Therapeutic Targeting Decreases Pancreatic Cancer Immunosuppression. Cancers 2022, 14, 4265.Cancers (Basel). 2022 Dec 14;14(24):6160. doi: 10.3390/cancers14246160. Cancers (Basel). 2022. PMID: 36551754 Free PMC article.
Abstract
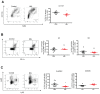
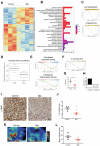
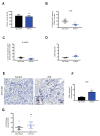
Background: The pancreatic ductal adenocarcinoma (PDAC) microenvironment is highly fibrotic and hypoxic, with poor immune cell infiltration. Recently, we showed that nucleolin (NCL) inhibition normalizes tumour vessels and impairs PDAC growth. Methods: Immunocompetent mouse models of PDAC were treated by the pseudopeptide N6L, which selectively inhibits NCL. Tumour-infiltrating immune cells and changes in the tumour microenvironment were analysed. Results: N6L reduced the proportion of regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs) and increased tumour-infiltrated T lymphocytes (TILs) with an activated phenotype. Low-dose anti-VEGFR2 treatment normalized PDAC vessels but did not modulate the immune suppressive microenvironment. RNAseq analysis of N6L-treated PDAC tumours revealed a reduction of cancer-associated fibroblast (CAF) expansion in vivo and in vitro. Notably, N6L treatment decreased IL-6 levels both in tumour tissues and in serum. Treating mPDAC by an antibody blocking IL-6 reduced the proportion of Tregs and MDSCs and increased the amount of TILs, thus mimicking the effects of N6L. Conclusions: These results demonstrate that NCL inhibition blocks the amplification of lymphoid and myeloid immunosuppressive cells and promotes T cell activation in PDAC through a new mechanism of action dependent on the direct inhibition of the tumoral stroma.
Keywords: PDAC; immune cells; nucleolin; vessels.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

