Bone Health Management in the Continuum of Prostate Cancer Disease
- PMID: 36077840
- PMCID: PMC9455007
- DOI: 10.3390/cancers14174305
Bone Health Management in the Continuum of Prostate Cancer Disease
Abstract
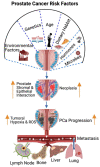
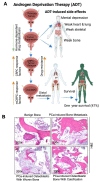
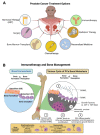
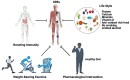
Prostate cancer (PCa) is the second-leading cause of cancer-related deaths in men. PCa cells require androgen receptor (AR) signaling for their growth and survival. Androgen deprivation therapy (ADT) is the preferred treatment for patients with locally advanced and metastatic PCa disease. Despite their initial response to androgen blockade, most patients eventually will develop metastatic castration-resistant prostate cancer (mCRPC). Bone metastases are common in men with mCRPC, occurring in 30% of patients within 2 years of castration resistance and in >90% of patients over the course of the disease. Patients with mCRPC-induced bone metastasis develop lesions throughout their skeleton; the 5-year survival rate for these patients is 47%. Bone-metastasis-induced early changes in the bone that proceed the osteoblastic response in the bone matrix are monitored and detected via modern magnetic resonance and PET/CT imaging technologies. Various treatment options, such as targeting osteolytic metastasis with bisphosphonates, prednisone, dexamethasone, denosumab, immunotherapy, external beam radiation therapy, radiopharmaceuticals, surgery, and pain medications are employed to treat prostate-cancer-induced bone metastasis and manage bone health. However, these diagnostics and treatment options are not very accurate nor efficient enough to treat bone metastases and manage bone health. In this review, we present the pathogenesis of PCa-induced bone metastasis, its deleterious impacts on vital organs, the impact of metastatic PCa on bone health, treatment interventions for bone metastasis and management of bone- and skeletal-related events, and possible current and future therapeutic options for bone management in the continuum of prostate cancer disease.
Keywords: androgen receptor; bisphosphonate; castration-resistant prostate cancer; denosumab; osteoblast; osteoclast; prostate cancer; radium-223; taxane.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Zheng Y., Lin S.X., Wu S., Dahl D.M., Blute M.L., Zhong W.D., Zhou X., Wu C.L. Clinicopathological characteristics of localized prostate cancer in younger men aged ≤ 50 years treated with radical prostatectomy in the PSA era: A systematic review and meta-analysis. Cancer Med. 2020;9:6473–6484. doi: 10.1002/cam4.3320. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

