Exo-Erythrocytic Development of Avian Haemosporidian Parasites in European Owls
- PMID: 36077935
- PMCID: PMC9454416
- DOI: 10.3390/ani12172212
Exo-Erythrocytic Development of Avian Haemosporidian Parasites in European Owls
Abstract
Avian haemosporidian parasites (Haemosporida, Apicomplexa) are globally distributed and infect birds of many orders. These pathogens have been much investigated in domestic and wild passeriform birds, in which they are relatively easy to access. In birds belonging to other orders, including owls (order Strigiformes), these parasites have been studied fragmentarily. Particularly little is known about the exo-erythrocytic development of avian haemosporidians. The goal of this study was to gain new knowledge about the parasites infecting owls in Europe and investigate their exo-erythrocytic stages. Tissue samples of 121 deceased owls were collected in Austria and Lithuania, and examined using polymerase chain reactions (PCR), histology, and chromogenic in situ hybridization (CISH). PCR-based diagnostics showed a total prevalence of 73.6%, revealing two previously unreported Haemoproteus and five novel Leucocytozoon lineages. By CISH and histology, meronts of several Leucocytozoon lineages (lASOT06, lSTAL5, lSTAL7) were discovered in the brains, heart muscles, and kidneys of infected birds. Further, megalomeronts of Haemoproteus syrnii (lineage hSTAL2) were discovered. This study contributes new knowledge to a better understanding of the biodiversity of avian haemosporidian parasites infecting owls in Europe, provides information on tissue stages of the parasites, and calls for further research of these under-investigated pathogens relevant to bird health.
Keywords: Strigiformes; birds; exo-erythrocytic development; haemosporidian parasites; tissue stages.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
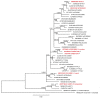

 )—meront, short black arrow (
)—meront, short black arrow ( )—cytomere, black arrowhead (
)—cytomere, black arrowhead ( )—merozoite, open arrowhead (
)—merozoite, open arrowhead ( )—intracellular young parasite stages, open arrow (
)—intracellular young parasite stages, open arrow ( )—meront containing capillary, long white arrow (
)—meront containing capillary, long white arrow ( )—erythrocytes, short white arrow (
)—erythrocytes, short white arrow ( )—host cell nucleus, white arrowhead (
)—host cell nucleus, white arrowhead ( )—wall. All scale bars are 25 µm.
)—wall. All scale bars are 25 µm.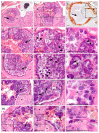
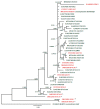
 )—meront, short black arrow (
)—meront, short black arrow ( )—cytomere, black arrowhead (
)—cytomere, black arrowhead ( )—merozoite, open arrowhead (
)—merozoite, open arrowhead ( )—intracellular young parasite stages, long white arrow (
)—intracellular young parasite stages, long white arrow ( )—erythrocytes, short white arrow (
)—erythrocytes, short white arrow ( )—host cell nucleus, white arrowhead (
)—host cell nucleus, white arrowhead ( )—wall, asterisk (
)—wall, asterisk ( )—smooth muscle layer. Scale bars are 25 µm.
)—smooth muscle layer. Scale bars are 25 µm.
 )—meront, white arrowhead (
)—meront, white arrowhead ( )—wall. Scale bars are 25 µm.
)—wall. Scale bars are 25 µm.
 )—megalomeront, short black arrow (
)—megalomeront, short black arrow ( )—cytomere, white arrowhead (
)—cytomere, white arrowhead ( )—wall, asterisk (
)—wall, asterisk ( )—necrosis. Scale bars are 50 µm.
)—necrosis. Scale bars are 50 µm.References
-
- Valkiūnas G., Atkinson C.T. Introduction to life cycles, taxonomy, distribution, and basic research techniques. In: Santiago-Alarcon D., Marzal A., editors. Avian Malaria and Related Parasites in the Tropics: Ecology, Evolution and Systematics. Springer Nature; Cham, Switzerland: 2020. pp. 45–80. - DOI
-
- Valkiūnas G. Avian Malaria Parasites and Other Haemosporidia. CRC Press; Boca Raton, FL, USA: 2005.
Grants and funding
LinkOut - more resources
Full Text Sources

