Effects of Para-Toluenesulfonamide on Canine Melanoma Xenotransplants in a BALB/c Nude Mouse Model
- PMID: 36077992
- PMCID: PMC9454485
- DOI: 10.3390/ani12172272
Effects of Para-Toluenesulfonamide on Canine Melanoma Xenotransplants in a BALB/c Nude Mouse Model
Abstract
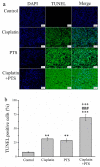
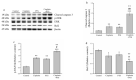
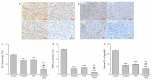
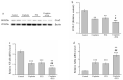
The pharmacological pathway of para-toluenesulfonamide (PTS) restricts the kinase activity of the mammalian target of rapamycin, potentially leading to reductions in cell division, cell growth, cell proliferation, and inflammation. These pathways have a critical effect on tumorigenesis. We aimed to examine the antitumor effect of PTS or PTS combined with cisplatin on canine melanoma implanted in BALB/c nude mice by estimating tumor growth, apoptosis expression, inflammation, and metastasis. The mice were randomly divided into four groups: control, cisplatin, PTS, and PTS combined with cisplatin. Mice treated with PTS or PTS combined with cisplatin had retarded tumor growth and increased tumor apoptosis through the enhanced expression of cleaved caspase 3 and extracellular signal-regulated kinase phosphorylation, decreased inflammatory cytokine levels, reduced inflammation-related factors, enhanced anti-inflammation-related factors, and inhibition of metastasis-related factors. Mice treated with PTS combined with cisplatin exhibited significantly retarded tumor growth, reduced tumor size, and increased tumor inhibition compared with those treated with cisplatin or PTS alone. PTS or PTS combined with cisplatin could retard canine melanoma growth and inhibit tumorigenesis. PTS and cisplatin were found to have an obvious synergistic tumor-inhibiting effect on canine melanoma. PTS alone and PTS combined with cisplatin may be antitumor agents for canine melanoma treatment.
Keywords: apoptosis; canine melanoma; cisplatin; inflammation; metastasis; para-toluenesulfonamide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Boria P.A., Murry D.J., Bennett P.F., Glickman N.W., Snyder P.W., Merkel B.L., Schlittler D.L., Mutsaers A.J., Thomas R.M., Knapp D.W. Evaluation of cisplatin combined with piroxicam for the treatment of oral malignant melanoma and oral squamous cell carcinoma in dogs. J. Am. Vet. Med. Assoc. 2004;224:388–394. doi: 10.2460/javma.2004.224.388. - DOI - PubMed
-
- Bergman P.J., Kent M.S., Farese J.P. Melanoma. In: Withrow S., Vail D., Page R., editors. Withrow and MacEwen’s Small Animal Clinical Oncology. Elsevier Saunders; St. Louis, MO, USA: 2013. pp. 321–333.
-
- Veena P., Kokila S., Rayadurgam V., Kumar S., Sankar P., Dhanalakshmi N. Malignant melanoma in a Dog—A Case report. Vet. World. 2012;5:431–432. doi: 10.5455/vetworld.2012.431-432. - DOI
-
- Atherton M.J., Morris J.S., McDermott M.R., Lichty B.D. Cancer immunology and canine malignant melanoma: A comparative review. Vet. Immunol. Immunopathol. 2016;169:15–26. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

