Nicotinamide Adenine Dinucleotide (NAD) Metabolism as a Relevant Target in Cancer
- PMID: 36078035
- PMCID: PMC9454445
- DOI: 10.3390/cells11172627
Nicotinamide Adenine Dinucleotide (NAD) Metabolism as a Relevant Target in Cancer
Abstract
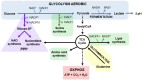
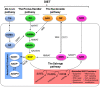
NAD+ is an important metabolite in cell homeostasis that acts as an essential cofactor in oxidation-reduction (redox) reactions in various energy production processes, such as the Krebs cycle, fatty acid oxidation, glycolysis and serine biosynthesis. Furthermore, high NAD+ levels are required since they also participate in many other nonredox molecular processes, such as DNA repair, posttranslational modifications, cell signalling, senescence, inflammatory responses and apoptosis. In these nonredox reactions, NAD+ is an ADP-ribose donor for enzymes such as sirtuins (SIRTs), poly-(ADP-ribose) polymerases (PARPs) and cyclic ADP-ribose (cADPRs). Therefore, to meet both redox and nonredox NAD+ demands, tumour cells must maintain high NAD+ levels, enhancing their synthesis mainly through the salvage pathway. NAMPT, the rate-limiting enzyme of this pathway, has been identified as an oncogene in some cancer types. Thus, NAMPT has been proposed as a suitable target for cancer therapy. NAMPT inhibition causes the depletion of NAD+ content in the cell, leading to the inhibition of ATP synthesis. This effect can cause a decrease in tumour cell proliferation and cell death, mainly by apoptosis. Therefore, in recent years, many specific inhibitors of NAMPT have been developed, and some of them are currently in clinical trials. Here we review the NAD metabolism as a cancer therapy target.
Keywords: NAD metabolism; cancer; nicotinamide adenine dinucleotide; therapeutic target.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Poljsak B. NAD+ in Cancer Prevention and Treatment: Pros and Cons. J. Clin. Exp. Oncol. 2016;4:2. doi: 10.4172/2324-9110.1000165. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

