Noradrenaline and Movement Initiation Disorders in Parkinson's Disease: A Pharmacological Functional MRI Study with Clonidine
- PMID: 36078048
- PMCID: PMC9454805
- DOI: 10.3390/cells11172640
Noradrenaline and Movement Initiation Disorders in Parkinson's Disease: A Pharmacological Functional MRI Study with Clonidine
Abstract
Slowness of movement initiation is a cardinal motor feature of Parkinson's disease (PD) and is not fully reverted by current dopaminergic treatments. This trouble could be due to the dysfunction of executive processes and, in particular, of inhibitory control of response initiation, a function possibly associated with the noradrenergic (NA) system. The implication of NA in the network supporting proactive inhibition remains to be elucidated using pharmacological protocols. For that purpose, we administered 150 μg of clonidine to 15 healthy subjects and 12 parkinsonian patients in a double-blind, randomized, placebo-controlled design. Proactive inhibition was assessed by means of a Go/noGo task, while pre-stimulus brain activity was measured by event-related functional MRI. Acute reduction in noradrenergic transmission induced by clonidine enhanced difficulties initiating movements reflected by an increase in omission errors and modulated the activity of the anterior node of the proactive inhibitory network (dorsomedial prefrontal and anterior cingulate cortices) in PD patients. We conclude that NA contributes to movement initiation by acting on proactive inhibitory control via the α2-adrenoceptor. We suggest that targeting noradrenergic dysfunction may represent a new treatment approach in some of the movement initiation disorders seen in Parkinson's disease.
Keywords: Parkinson’s disease; akinesia; clonidine; functional MRI; inhibitory control; movement initiation; noradrenaline; α2-adrenoceptor.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
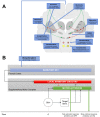
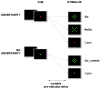
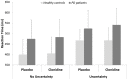
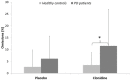

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

