Physiological Mineralization during In Vitro Osteogenesis in a Biomimetic Spheroid Culture Model
- PMID: 36078105
- PMCID: PMC9454617
- DOI: 10.3390/cells11172702
Physiological Mineralization during In Vitro Osteogenesis in a Biomimetic Spheroid Culture Model
Abstract
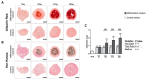
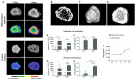
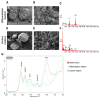
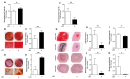
Bone health-targeting drug development strategies still largely rely on inferior 2D in vitro screenings. We aimed at developing a scaffold-free progenitor cell-based 3D biomineralization model for more physiological high-throughput screenings. MC3T3-E1 pre-osteoblasts were cultured in α-MEM with 10% FCS, at 37 °C and 5% CO2 for up to 28 days, in non-adherent V-shaped plates to form uniformly sized 3D spheroids. Osteogenic differentiation was induced by 10 mM β-glycerophosphate and 50 µg/mL ascorbic acid. Mineralization stages were assessed through studying expression of marker genes, alkaline phosphatase activity, and calcium deposition by histochemistry. Mineralization quality was evaluated by Fourier transformed infrared (FTIR) and scanning electron microscopic (SEM) analyses and quantified by micro-CT analyses. Expression profiles of selected early- and late-stage osteoblast differentiation markers indicated a well-developed 3D biomineralization process with strongly upregulated Col1a1, Bglap and Alpl mRNA levels and type I collagen- and osteocalcin-positive immunohistochemistry (IHC). A dynamic biomineralization process with increasing mineral densities was observed during the second half of the culture period. SEM-Energy-Dispersive X-ray analyses (EDX) and FTIR ultimately confirmed a native bone-like hydroxyapatite mineral deposition ex vivo. We thus established a robust and versatile biomimetic, and high-throughput compatible, cost-efficient spheroid culture model with a native bone-like mineralization for improved pharmacological ex vivo screenings.
Keywords: Fourier-transform infrared spectroscopy (FT-IR); MC3T3-E1; osteoblast progenitors; osteogenic differentiation; physiological biomineralization; spheroid culture; volumetric micro-CT quantification.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


Similar articles
-
von Kossa staining alone is not sufficient to confirm that mineralization in vitro represents bone formation.Calcif Tissue Int. 2003 May;72(5):537-47. doi: 10.1007/s00223-002-1057-y. Epub 2003 May 6. Calcif Tissue Int. 2003. PMID: 12724828
-
Extracellular matrix mineralization in murine MC3T3-E1 osteoblast cultures: an ultrastructural, compositional and comparative analysis with mouse bone.Bone. 2015 Feb;71:244-56. doi: 10.1016/j.bone.2014.11.003. Epub 2014 Nov 13. Bone. 2015. PMID: 25460184 Free PMC article.
-
Bone morphogenetic protein-2 restores mineralization in glucocorticoid-inhibited MC3T3-E1 osteoblast cultures.J Bone Miner Res. 2003 Jul;18(7):1186-97. doi: 10.1359/jbmr.2003.18.7.1186. J Bone Miner Res. 2003. PMID: 12854828
-
In vitro mineralization of MC3T3-E1 osteoblast-like cells on collagen/nano-hydroxyapatite scaffolds coated carbon/carbon composites.J Biomed Mater Res A. 2016 Feb;104(2):533-43. doi: 10.1002/jbm.a.35593. Epub 2015 Nov 3. J Biomed Mater Res A. 2016. PMID: 26476098
-
Spheroids as a 3D in vitro model to study bone and bone mineralization.Biomater Adv. 2024 Feb;157:213727. doi: 10.1016/j.bioadv.2023.213727. Epub 2023 Dec 10. Biomater Adv. 2024. PMID: 38101067 Review.
Cited by
-
In silico engineering and simulation of RNA interferences nanoplatforms for osteoporosis treating and bone healing promoting.Sci Rep. 2023 Oct 24;13(1):18185. doi: 10.1038/s41598-023-45183-3. Sci Rep. 2023. PMID: 37875547 Free PMC article.
-
Three-Dimensional Cell Cultures: The Bridge between In Vitro and In Vivo Models.Int J Mol Sci. 2023 Jul 27;24(15):12046. doi: 10.3390/ijms241512046. Int J Mol Sci. 2023. PMID: 37569426 Free PMC article. Review.
-
Inducing Osteogenesis in Human Pulp Stem Cells Cultured on Nano-Hydroxyapatite and Naringin-Coated 3D-Printed Poly Lactic Acid Scaffolds.Polymers (Basel). 2025 Feb 24;17(5):596. doi: 10.3390/polym17050596. Polymers (Basel). 2025. PMID: 40076089 Free PMC article.
-
Hypoxia-Preconditioned Human Umbilical Cord Mesenchymal Stem Cells Transplantation Ameliorates Inflammation and Bone Regeneration in Peri-Implantitis Rat Model.Eur J Dent. 2025 May;19(2):420-427. doi: 10.1055/s-0044-1791530. Epub 2024 Nov 7. Eur J Dent. 2025. PMID: 39510521 Free PMC article.
-
Matrix Stiffness Regulates the Osteogenic Differentiation of hPDLSCs via DNA Methylation.Int Dent J. 2025 Aug;75(4):100783. doi: 10.1016/j.identj.2025.02.022. Epub 2025 May 1. Int Dent J. 2025. PMID: 40315698 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

