Current Understanding of Asthma Pathogenesis and Biomarkers
- PMID: 36078171
- PMCID: PMC9454904
- DOI: 10.3390/cells11172764
Current Understanding of Asthma Pathogenesis and Biomarkers
Abstract
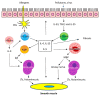
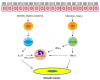
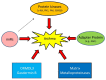
Asthma is a heterogeneous lung disease with variable phenotypes (clinical presentations) and distinctive endotypes (mechanisms). Over the last decade, considerable efforts have been made to dissect the cellular and molecular mechanisms of asthma. Aberrant T helper type 2 (Th2) inflammation is the most important pathological process for asthma, which is mediated by Th2 cytokines, such as interleukin (IL)-5, IL-4, and IL-13. Approximately 50% of mild-to-moderate asthma and a large portion of severe asthma is induced by Th2-dependent inflammation. Th2-low asthma can be mediated by non-Th2 cytokines, including IL-17 and tumor necrosis factor-α. There is emerging evidence to demonstrate that inflammation-independent processes also contribute to asthma pathogenesis. Protein kinases, adapter protein, microRNAs, ORMDL3, and gasdermin B are newly identified molecules that drive asthma progression, independent of inflammation. Eosinophils, IgE, fractional exhaled nitric oxide, and periostin are practical biomarkers for Th2-high asthma. Sputum neutrophils are easily used to diagnose Th2-low asthma. Despite progress, more studies are needed to delineate complex endotypes of asthma and to identify new and practical biomarkers for better diagnosis, classification, and treatment.
Keywords: asthma; biomarker; cytokine; inflammation; smooth muscle.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

