Novel Chimeric Vaccine Candidate Development against Leptotrichia buccalis
- PMID: 36078462
- PMCID: PMC9518150
- DOI: 10.3390/ijerph191710742
Novel Chimeric Vaccine Candidate Development against Leptotrichia buccalis
Abstract
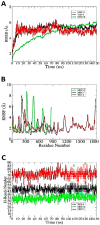
The misuse of antibiotics in our daily lives has led to the emergence of antimicrobial resistance. As a result, many antibiotics are becoming ineffective. This phenomenon is linked with high rates of mortality and morbidity. Therefore, new approaches are required to address this major health issue. Leptotrichia buccalis is a Gram-negative, rod-shaped bacterium which normally resides in the oral and vaginal cavities. It is an emerging bacterial pathogen which is developing new antibiotic-resistance mechanisms. No approved vaccine is available against this pathogen, which is a cause for growing concern. In this study, an in silico-based, multi-epitopes vaccine against this pathogen was designed by applying reverse vaccinology and immunoinformatic approaches. Of a total of 2193 predicted proteins, 294 were found to be redundant while 1899 were non-redundant. Among the non-redundant proteins, 6 were predicted to be present in the extracellular region, 12 in the periplasmic region and 23 in the outer-membrane region. Three proteins (trypsin-like peptidase domain-containing protein, sel1 repeat family protein and TrbI/VirB10 family protein) were predicted to be virulent and potential subunit vaccine targets. In the epitopes prediction phase, the three proteins were subjected to B- and T-cell epitope mapping; 19 epitopes were used for vaccine design. The vaccine construct was docked with MHC-I, MHC-II and TLR-4 immune receptors and only the top-ranked complex (based on global energy value) was selected in each case. The selected docked complexes were examined in a molecular dynamic simulation and binding free energies analysis in order to assess their intermolecular stability. It was observed that the vaccine binding mode with receptors was stable and that the system presented stable dynamics. The net binding free energy of complexes was in the range of -300 to -500 kcal/mol, indicating the formation of stable complexes. In conclusion, the data reported herein might help vaccinologists to formulate a chimeric vaccine against the aforementioned target pathogen.
Keywords: Leptotrichia buccalis; molecular docking; molecular dynamics simulation; multi-epitopes vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
A computational quest for identifying potential vaccine candidates against Moraxella lacunata: a multi-pronged approach.J Biomol Struct Dyn. 2024 Apr;42(6):2976-2989. doi: 10.1080/07391102.2023.2212793. Epub 2023 May 12. J Biomol Struct Dyn. 2024. PMID: 37177816
-
An In Silico Multi-epitopes Vaccine Ensemble and Characterization Against Nosocomial Proteus penneri.Mol Biotechnol. 2024 Dec;66(12):3498-3513. doi: 10.1007/s12033-023-00949-y. Epub 2023 Nov 7. Mol Biotechnol. 2024. PMID: 37934390
-
Computational exploration and design of a multi-epitopes vaccine construct against Chlamydia psittaci.J Biomol Struct Dyn. 2024;42(22):12105-12121. doi: 10.1080/07391102.2023.2268173. Epub 2023 Oct 28. J Biomol Struct Dyn. 2024. PMID: 37897717
-
Computational study to investigate Proteus mirabilis proteomes for multi-epitope vaccine construct design.J Biomol Struct Dyn. 2023 Nov;41(19):10190-10201. doi: 10.1080/07391102.2022.2153920. Epub 2022 Dec 7. J Biomol Struct Dyn. 2023. PMID: 36476074
-
Computational Design of a Chimeric Vaccine against Plesiomonas shigelloides Using Pan-Genome and Reverse Vaccinology.Vaccines (Basel). 2022 Nov 8;10(11):1886. doi: 10.3390/vaccines10111886. Vaccines (Basel). 2022. PMID: 36366394 Free PMC article.
Cited by
-
Preparation of Acetylcholinesterase Inhibitory Peptides from Yellowfin Tuna Pancreas Using Moderate Ultrasound-Assisted Enzymatic Hydrolysis.Mar Drugs. 2025 Feb 9;23(2):75. doi: 10.3390/md23020075. Mar Drugs. 2025. PMID: 39997199 Free PMC article.
-
Design of an Epitope-Based Vaccine Against MERS-CoV.Medicina (Kaunas). 2024 Oct 6;60(10):1632. doi: 10.3390/medicina60101632. Medicina (Kaunas). 2024. PMID: 39459420 Free PMC article.
-
Chimeric vaccine design against the conserved TonB-dependent receptor-like β-barrel domain from the outer membrane tbpA and hpuB proteins of Kingella kingae ATCC 23330.Front Mol Biosci. 2023 Nov 20;10:1258834. doi: 10.3389/fmolb.2023.1258834. eCollection 2023. Front Mol Biosci. 2023. PMID: 38053576 Free PMC article.
-
Exploring glutathione transferase and Cathepsin L-like proteinase for designing of epitopes-based vaccine against Fasciola hepatica by immunoinformatics and biophysics studies.Front Immunol. 2024 Sep 26;15:1478107. doi: 10.3389/fimmu.2024.1478107. eCollection 2024. Front Immunol. 2024. PMID: 39391319 Free PMC article.
-
A novel vaccine construct against Zika virus fever: insights from epitope-based vaccine discovery through molecular modeling and immunoinformatics approaches.Front Immunol. 2024 Jul 1;15:1426496. doi: 10.3389/fimmu.2024.1426496. eCollection 2024. Front Immunol. 2024. PMID: 39050858 Free PMC article.
References
-
- Fonti V., Di Cesare A., Šangulin J., Del Negro P., Celussi M. Antibiotic Resistance Genes and Potentially Pathogenic Bacteria in the Central Adriatic Sea: Are They Connected to Urban Wastewater Inputs? Water. 2021;13:3335. doi: 10.3390/w13233335. - DOI
-
- Tacconelli E., Carrara E., Savoldi A., Harbarth S., Mendelson M., Monnet D.L., Pulcini C., Kahlmeter G., Kluytmans J., Carmeli Y., et al. Discovery, Research, and Development of New Antibiotics: The WHO Priority List of Antibiotic-Resistant Bacteria and Tuberculosis. Lancet Infect. Dis. 2018;18:318–327. doi: 10.1016/S1473-3099(17)30753-3. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials

