Measuring T-Cell Responses against SARS-CoV-2 Is of Utility for Disease and Vaccination Management
- PMID: 36079033
- PMCID: PMC9457376
- DOI: 10.3390/jcm11175103
Measuring T-Cell Responses against SARS-CoV-2 Is of Utility for Disease and Vaccination Management
Abstract
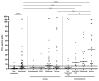
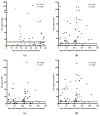
The measurement of specific T-cell responses can be a useful tool for COVID-19 diagnostics and clinical management. In this study, we evaluated the IFN-γ T-cell response against the main SARS-CoV-2 antigens (spike, nucleocapsid and membrane) in acute and convalescent individuals classified according to severity, and in vaccinated and unvaccinated controls. IgG against spike and nucleocapsid were also measured. Spike antigen triggered the highest number of T-cell responses. Acute patients showed a low percentage of positive responses when compared to convalescent (71.6% vs. 91.7%, respectively), but increased during hospitalization and with severity. Some convalescent patients showed an IFN-γ T-cell response more than 200 days after diagnosis. Only half of the vaccinated individuals displayed an IFN-γ T-cell response after the second dose. IgG response was found in a higher percentage of individuals compared to IFN-γ T-cell responses, and moderate correlations between both responses were seen. However, in some acute COVID-19 patients specific T-cell response was detected, but not IgG production. We found that the chances of an IFN-γ T-cell response against SARS-CoV-2 is low during acute phase, but may increase over time, and that only half of the vaccinated individuals had an IFN-γ T-cell response after the second dose.
Keywords: ELISPOT; IFN-γ; SARS-CoV-2; T-cell response; humoral response; vaccination.
Conflict of interest statement
The authors declare no conflict of interest. The funders did not play a role in the study design, conduct, collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Figures

References
-
- WHO Coronavirus (COVID-19) Dashboard. [(accessed on 12 July 2022)]. Available online: https://covid19.who.int.
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

