Advances in Optical Coherence Tomography Imaging Technology and Techniques for Choroidal and Retinal Disorders
- PMID: 36079077
- PMCID: PMC9457394
- DOI: 10.3390/jcm11175139
Advances in Optical Coherence Tomography Imaging Technology and Techniques for Choroidal and Retinal Disorders
Abstract
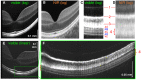
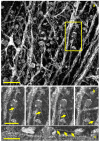
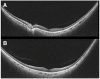
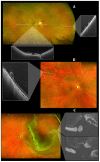
Optical coherence tomography (OCT) imaging has played a pivotal role in the field of retina. This light-based, non-invasive imaging modality provides high-quality, cross-sectional analysis of the retina and has revolutionized the diagnosis and management of retinal and choroidal diseases. Since its introduction in the early 1990s, OCT technology has continued to advance to provide quicker acquisition times and higher resolution. In this manuscript, we discuss some of the most recent advances in OCT technology and techniques for choroidal and retinal diseases. The emerging innovations discussed include wide-field OCT, adaptive optics OCT, polarization sensitive OCT, full-field OCT, hand-held OCT, intraoperative OCT, at-home OCT, and more. The applications of these rising OCT systems and techniques will allow for a closer monitoring of chorioretinal diseases and treatment response, more robust analysis in basic science research, and further insights into surgical management. In addition, these innovations to optimize visualization of the choroid and retina offer a promising future for advancing our understanding of the pathophysiology of chorioretinal diseases.
Keywords: advances; choroid; optical coherence tomography; retina; technology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Majumdar S., Tripathy K. StatPearls. StatPearls; Treasure Island, FL, USA: 2022. Macular Hole. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

