Switching between Enzyme Replacement Therapies and Substrate Reduction Therapies in Patients with Gaucher Disease: Data from the Gaucher Outcome Survey (GOS)
- PMID: 36079085
- PMCID: PMC9457166
- DOI: 10.3390/jcm11175158
Switching between Enzyme Replacement Therapies and Substrate Reduction Therapies in Patients with Gaucher Disease: Data from the Gaucher Outcome Survey (GOS)
Abstract
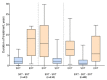
Switching between enzyme replacement therapies (ERT) and substrate reduction therapies (SRT) in patients with type 1 Gaucher disease (GD1) is not uncommon; however, the reasons for switchng treatments have not been explored in detail. Data from the Gaucher Outcome Survey (GOS), an international registry for patients with confirmed GD, were used to evaluate the reasons for, and consequences of, switching between these treatment types. Of the 1843 patients enrolled in GOS on 25 February 2020, 245 had undergone a treatment switch: 222 from initial ERT to SRT (of whom 88 later switched back to ERT) and 23 from initial SRT to ERT. The most common reasons for ERT-SRT switching were duration of infusion (25.4%), drug shortage (22.0%), and adverse events (AEs; 11.9%), and for SRT-ERT switching, AEs (63.6%), lack of beneficial effect (16.4%), and participation in a clinical trial (9.1%). Bodyweight and hematologic parameters largely remained stable before and after switching between ERT and SRT, although with substantial variation between patients. These findings contribute to understanding why treatment switching occurs in patients with GD, and may help physicians recognize the real-world impact of treatment switching between ERT and SRT for patients with GD.
Keywords: Gaucher disease; enzyme replacement therapy; substrate reduction therapy; treatment switch.
Conflict of interest statement
D.A.H has received consulting fees and fees for non-CME/CE services from Genzyme, Sanofi, and Takeda; P.D. has received speaker and board membership fees from Takeda and consulting fees from Sanofi; P.G. has received fees for presentations and advisory boards and grants for research projects from Alexion, Amicus, Pfizer, Sanofi Genzyme, and Takeda; O.G.-A. has served on advisory boards and received consulting fees from 4DMT, Amicus, Sangamo, Sanofi Genzyme, and Takeda, has research contracts with 4DMT, Amicus, AVROBIO, Freeline, Genentech, Protalix, Sangamo, Sanofi Genzyme, and Takeda, and has participated in speaker bureaus for Sanofi Genzyme and Takeda; H.L. has received consulting fees from Actelion, Pfizer, Sanofi Genzyme, and Takeda, and research grants from Amicus, BioMarin, Pfizer, Protalix, Sangamo, Sanofi Genzyme, Takeda, and Ultragenyx; E.L. has received honoraria and travel reimbursement from Sanofi Genzyme and Shire (now Takeda); S.R.-V. has received speaker fees and travel support from Pfizer, Sanofi Genzyme, and Takeda; M.S. has received honoraria and travel support from Alexion, BioMarin, CHIESI, Orchard, Regenxbio, Sanofi, Takeda, and Ultragenyx; J.B. and N.G. are employees of Takeda; A.Z. has received honoraria from BioEvents, Pfizer, and Takeda, and consulting fees from Avrobio (at the time the analysis took place), Insightec, Prevail Therapeutics, and Takeda.
Figures



References
-
- Revel-Vilk S., Szer J., Zimran A. Gaucher disease and related lysosomal storage diseases. In: Kaushansky K., Lichtman M., Prchal J., Levi M., Burns L.J., Linch D., editors. Williams Hematology. 10th ed. McGraw-Hill; New York, NY, USA: 2021.
-
- Zimran A., Durán G., Giraldo P., Rosenbaum H., Giona F., Petakov M., Terreros Muñoz E., Solorio-Meza S.E., Cooper P.A., Varughese S., et al. Long-term efficacy and safety results of taliglucerase alfa through 5years in adult treatment-naïve patients with Gaucher disease. Blood Cells Mol. Dis. 2019;78:14–21. doi: 10.1016/j.bcmd.2016.07.002. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

