Machine Perfusion for Extended Criteria Donor Livers: What Challenges Remain?
- PMID: 36079148
- PMCID: PMC9457017
- DOI: 10.3390/jcm11175218
Machine Perfusion for Extended Criteria Donor Livers: What Challenges Remain?
Abstract
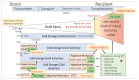
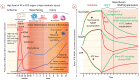
Based on the renaissance of dynamic preservation techniques, extended criteria donor (ECD) livers reclaimed a valuable eligibility in the transplantable organ pool. Being more vulnerable to ischemia, ECD livers carry an increased risk of early allograft dysfunction, primary non-function and biliary complications and, hence, unveiled the limitations of static cold storage (SCS). There is growing evidence that dynamic preservation techniques-dissimilar to SCS-mitigate reperfusion injury by reconditioning organs prior transplantation and therefore represent a useful platform to assess viability. Yet, a debate is ongoing about the advantages and disadvantages of different perfusion strategies and their best possible applications for specific categories of marginal livers, including organs from donors after circulatory death (DCD) and brain death (DBD) with extended criteria, split livers and steatotic grafts. This review critically discusses the current clinical spectrum of livers from ECD donors together with the various challenges and posttransplant outcomes in the context of standard cold storage preservation. Based on this, the potential role of machine perfusion techniques is highlighted next. Finally, future perspectives focusing on how to achieve higher utilization rates of the available donor pool are highlighted.
Keywords: donation after circulatory death; extended criteria donors; machine perfusion; mitochondria.
Conflict of interest statement
The authors declare no conflict of interest related to this article.
Figures



References
-
- Brettschneider L., Daloze P.M., Huguet C., Porter K.A., Groth C.G., Kashiwagi N., Hutchison D.E., Starzl T.E. The use of combined preservation techniques for extended storage of orthotopic liver homografts. Surg. Gynecol. Obstet. 1968;126:263–274. doi: 10.1097/00007890-196808000-00019. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

