Supramolecular Functionalisation of B/N Co-Doped Carbon Nano-Onions for Novel Nanocarrier Systems
- PMID: 36079368
- PMCID: PMC9456768
- DOI: 10.3390/ma15175987
Supramolecular Functionalisation of B/N Co-Doped Carbon Nano-Onions for Novel Nanocarrier Systems
Abstract
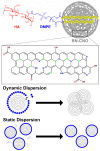
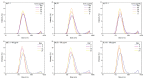
Boron/nitrogen co-doped carbon nano-onions (BN-CNOs) are spherical nanoparticles that consist of multiple inter-nestled fullerene layers, giving them an onion-like internal structure. They have potential as nanocarriers due to their small size, aqueous dispersibility, and biocompatibility. The non-covalent attachment of a biocompatible polymer to BN-CNOs is a simple and effective method of creating a scaffold for a novel nanocarrier system as it allows for increased aqueous dispersibility whilst preventing the immune system from recognising the particle as a foreign object. The non-covalent approach also preserves the electronic and structural properties of the BN-CNOs. In this study, we attached a hyaluronic acid-phospholipid (HA-DMPE) conjugate polymer to the BN-CNO's surface to improve its hydrophilicity and provide targetability toward HA-receptor overexpressing cancer cells. To this end, various ratios of HA-DMPE to BN-CNOs were investigated. The resulting supramolecular systems were characterised via UV-Vis absorption and FTIR spectroscopy, dynamic light scattering, and zeta potential techniques. It was found that the HA-DMPE conjugate polymer was permanently wrapped around the BN-CNO nanoparticle surface. Moreover, the resulting BN-CNO/HA-DMPE supramolecular systems displayed enhanced aqueous solubility compared to unfunctionalised BN-CNOs, with excellent long-term stability observed in aqueous dispersions.
Keywords: CD44; aqueous dispersibility; boron/nitrogen doping; carbon nano-onions; carbon nanomaterials; conjugate polymer; hyaluronic acid; nanocarrier; phospholipid; supramolecular system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
In Vitro and In Vivo Biocompatibility of Boron/Nitrogen Co-Doped Carbon Nano-Onions.Nanomaterials (Basel). 2021 Nov 10;11(11):3017. doi: 10.3390/nano11113017. Nanomaterials (Basel). 2021. PMID: 34835781 Free PMC article.
-
B/N-doped carbon nano-onions as nanocarriers for targeted breast cancer therapy.Nanoscale. 2025 May 15;17(19):12108-12123. doi: 10.1039/d4nr04990j. Nanoscale. 2025. PMID: 40183172
-
Supramolecular functionalization of carbon nano-onions with hyaluronic acid-phospholipid conjugates for selective targeting of cancer cells.Colloids Surf B Biointerfaces. 2020 Apr;188:110779. doi: 10.1016/j.colsurfb.2020.110779. Epub 2020 Jan 8. Colloids Surf B Biointerfaces. 2020. PMID: 31955017
-
Supramolecular chemistry of carbon nano-onions.Nanoscale. 2020 May 7;12(17):9352-9358. doi: 10.1039/d0nr01713b. Nanoscale. 2020. PMID: 32329483 Review.
-
Carbon nano-onions as potential nanocarriers for drug delivery.Dalton Trans. 2021 Feb 23;50(7):2300-2309. doi: 10.1039/d0dt04093b. Dalton Trans. 2021. PMID: 33471000 Review.
Cited by
-
Engineering of Green Carbon Dots for Biomedical and Biotechnological Applications.Molecules. 2024 Sep 23;29(18):4508. doi: 10.3390/molecules29184508. Molecules. 2024. PMID: 39339503 Free PMC article. Review.
-
Carbon Dot-Based Nanoparticles: A Promising Therapeutic Approach for Glioblastoma.Int J Nanomedicine. 2025 May 31;20:7061-7092. doi: 10.2147/IJN.S519733. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40487863 Free PMC article. Review.
-
Carbon Nanomaterials (CNMs) in Cancer Therapy: A Database of CNM-Based Nanocarrier Systems.Pharmaceutics. 2023 May 19;15(5):1545. doi: 10.3390/pharmaceutics15051545. Pharmaceutics. 2023. PMID: 37242787 Free PMC article. Review.
-
Nitrogen-doped carbon nano-onions/polypyrrole nanocomposite based low-cost flexible sensor for room temperature ammonia detection.Sci Rep. 2024 Apr 4;14(1):7904. doi: 10.1038/s41598-024-57153-4. Sci Rep. 2024. PMID: 38570517 Free PMC article.
References
-
- Bobrowska D.M., Czyrko J., Brzezinski K., Echegoyen L., Plonska-Brzezinska M.E. Carbon Nano-Onion Composites: Physicochemical Characteristics and Biological Activity. Fuller. Nanotub. Carbon Nanostructures. 2017;25:185–192. doi: 10.1080/1536383X.2016.1248758. - DOI
-
- Ugarte D. Onion-Like Graphitic Particles. In: Endo M., Iijima S., Dresselhaus M.S., editors. Carbon Nanotubes. Pergamon; Oxford, UK: 1996. pp. 163–167. - DOI
-
- Mordkovich V.Z., Takeuchi Y. Multishell Fullerenes by Laser Vaporization of Composite Carbon–Metal Targets. Chem. Phys. Lett. 2002;355:133–138. doi: 10.1016/S0009-2614(02)00196-3. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

