Pillar[n]arene-Mimicking/Assisted/Participated Carbon Nanotube Materials
- PMID: 36079500
- PMCID: PMC9458132
- DOI: 10.3390/ma15176119
Pillar[n]arene-Mimicking/Assisted/Participated Carbon Nanotube Materials
Abstract
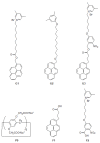
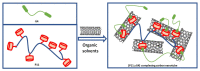
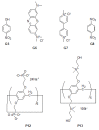
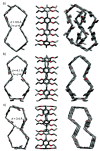
The recent progress in pillar[n]arene-assisted/participated carbon nanotube hybrid materials were initially summarized and discussed. The molecular structure of pillar[n]arene could serve different roles in the fabrication of attractive carbon nanotube-based materials. Firstly, pillar[n]arene has the ability to provide the structural basis for enlarging the cylindrical pillar-like architecture by forming one-dimensional, rigid, tubular, oligomeric/polymeric structures with aromatic moieties as the linker, or forming spatially "closed", channel-like, flexible structures by perfunctionalizing with peptides and with intramolecular hydrogen bonding. Interestingly, such pillar[n]arene-based carbon nanotube-resembling structures were used as porous materials for the adsorption and separation of gas and toxic pollutants, as well as for artificial water channels and membranes. In addition to the art of organic synthesis, self-assembly based on pillar[n]arene, such as self-assembled amphiphilic molecules, is also used to promote and control the dispersion behavior of carbon nanotubes in solution. Furthermore, functionalized pillar[n]arene derivatives integrated carbon nanotubes to prepare advanced hybrid materials through supramolecular interactions, which could also incorporate various compositions such as Ag and Au nanoparticles for catalysis and sensing.
Keywords: application; carbon nanotube; hybrid materials; pillar[n]arene; synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
Pillar[n]arene-calix[m]arene hybrid macrocyclic structures.RSC Adv. 2022 Oct 3;12(43):28185-28195. doi: 10.1039/d2ra05118d. eCollection 2022 Sep 28. RSC Adv. 2022. PMID: 36320255 Free PMC article. Review.
-
Pillar[5]arene-Decorated Single-Walled Carbon Nanotubes.ACS Omega. 2018 Oct 23;3(10):13935-13943. doi: 10.1021/acsomega.8b02091. eCollection 2018 Oct 31. ACS Omega. 2018. PMID: 31458090 Free PMC article.
-
Applications of Supramolecular Polymers Generated from Pillar[n]arene-Based Molecules.Polymers (Basel). 2023 Nov 27;15(23):4543. doi: 10.3390/polym15234543. Polymers (Basel). 2023. PMID: 38231964 Free PMC article. Review.
-
Cyclodextrin-pillar[n]arene hybridized macrocyclic systems.Org Biomol Chem. 2022 Jun 1;20(21):4278-4288. doi: 10.1039/d2ob00671e. Org Biomol Chem. 2022. PMID: 35552579 Review.
-
Fullerene-containing pillar[n]arene hybrid composites.Org Biomol Chem. 2022 Nov 2;20(42):8176-8186. doi: 10.1039/d2ob01664h. Org Biomol Chem. 2022. PMID: 36226561 Review.
Cited by
-
Applications of Advanced Nanomaterials in Sensor Devices.Materials (Basel). 2022 Dec 16;15(24):8995. doi: 10.3390/ma15248995. Materials (Basel). 2022. PMID: 36556798 Free PMC article.
References
-
- Driggers E.M., Hale S.P., Lee J., Terrett N.K. The exploration of macrocycles for drug discovery–an underexploited structural class. Nat. Rev. Drug Discov. 2008;7:608–624. - PubMed
-
- Wang Y., Wu H., Stoddart J.F. Molecular triangles: A new class of macrocycles. Acc. Chem. Res. 2021;54:2027–2039. - PubMed
-
- Liu Z.C., Nalluri S.K.M., Stoddart J.F. Surveying macrocyclic chemistry: From flexible crown ethers to rigid cyclophanes. Chem. Soc. Rev. 2017;46:2459–2478. - PubMed
-
- Lakshmanan V.I., Vijayan S. A review on application of crown ethers in separation of rare earths and precious metals. In: Davis B.R., Moats M.S., Wang S.J., editors. The Minerals, Metals & Materials Series. Springer; Mississauga, ON, Canada: 2018. pp. 1913–1930.
-
- Duan Z.Z., Xu F.F., Huang X.H., Qian Y.C., Li H., Tian W. Crown ether-based supramolecular polymers: From synthesis to self-assembly. Macromol. Rapid Commun. 2022;43:e2100775. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

