In Vitro and In Silico Screening of Anti- Vibrio spp., Antibiofilm, Antioxidant and Anti-Quorum Sensing Activities of Cuminum cyminum L. Volatile Oil
- PMID: 36079620
- PMCID: PMC9459890
- DOI: 10.3390/plants11172236
In Vitro and In Silico Screening of Anti- Vibrio spp., Antibiofilm, Antioxidant and Anti-Quorum Sensing Activities of Cuminum cyminum L. Volatile Oil
Abstract
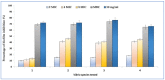
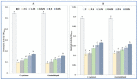
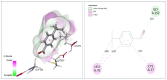
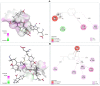
Cuminum cyminum L. essential oil (cumin EO) was studied for its chemical composition, antioxidant and vibriocidal activities. Inhibition of biofilm formation and secretion of some virulence properties controlled by the quorum sensing system in Chromobacterium violaceum and Pseudomonas aeruginosa strains were also reported. The obtained results showed that cuminaldehyde (44.2%) was the dominant compound followed by β-pinene (15.1%), γ-terpinene (14.4%), and p-cymene (14.2%). Using the disc diffusion assay, cumin EO (10 mg/disc) was particularly active against all fifteen Vibrio species, and the highest diameter of growth inhibition zone was recorded against Vibrio fluvialis (41.33 ± 1.15 mm), Vibrio parahaemolyticus (39.67 ± 0.58 mm), and Vibrio natrigens (36.67 ± 0.58 mm). At low concentration (MICs value from 0.023-0.046 mg/mL), cumin EO inhibited the growth of all Vibrio strains, and concentrations as low as 1.5 mg/mL were necessary to kill them (MBCs values from 1.5-12 mg/mL). Using four antioxidant assays, cumin EO exhibited a good result as compared to standard molecules (DPPH = 8 ± 0.54 mg/mL; reducing power = 3.5 ± 0.38 mg/mL; β-carotene = 3.8 ± 0.34 mg/mL; chelating power = 8.4 ± 0.14 mg/mL). More interestingly, at 2x MIC value, cumin EO inhibited the formation of biofilm by Vibrio alginolyticus (9.96 ± 1%), V. parahaemolyticus (15.45 ± 0.7%), Vibrio cholerae (14.9 ± 0.4%), and Vibrio vulnificus (18.14 ± 0.3%). In addition, cumin EO and cuminaldehyde inhibited the production of violacein on Lauria Bertani medium (19 mm and 35 mm, respectively). Meanwhile, 50% of violacein inhibition concentration (VIC50%) was about 2.746 mg/mL for cumin EO and 1.676 mg/mL for cuminaldehyde. Moreover, elastase and protease production and flagellar motility in P. aeruginosa were inhibited at low concentrations of cumin EO and cuminaldehyde. The adopted in-silico approach revealed good ADMET properties as well as a high binding score of the main compounds with target proteins (1JIJ, 2UV0, 1HD2, and 3QP1). Overall, the obtained results highlighted the effectiveness of cumin EO to prevent spoilage with Vibrio species and to interfere with the quorum sensing system in Gram-negative bacteria by inhibiting the flagellar motility, formation of biofilm, and the secretion of some virulence enzymes.
Keywords: Cuminum cyminum L.; Vibrio spp.; antioxidant; in silico approach; phytochemistry.
Conflict of interest statement
Authors declare that there are no conflict of interest.
Figures


References
-
- Dhingra S., Rahman N.A.A., Peile E., Rahman M., Sartelli M., Hassali M.A., Islam T., Islam S., Haque M. Microbial Resistance Movements: An Overview of Global Public Health Threats Posed by Antimicrobial Resistance, and How Best to Counter. Front. Public Health. 2020;8:535668. doi: 10.3389/fpubh.2020.535668. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

