A New Stilbene Glucoside from Biotransformation-Guided Purification of Chinese Herb Ha-Soo-Oh
- PMID: 36079669
- PMCID: PMC9460137
- DOI: 10.3390/plants11172286
A New Stilbene Glucoside from Biotransformation-Guided Purification of Chinese Herb Ha-Soo-Oh
Abstract
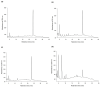
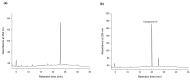
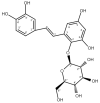
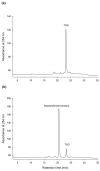
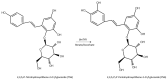
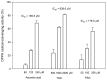
Ha-Soo-Oh is a traditional Chinese medicine prepared from the roots of Polygonum multiflorum Thunb. The herb extract has been widely used in Asian countries as a tonic agent and nutritional supplement for centuries. To identify new bioactive compounds in Chinese herbs, the biotransformation-guided purification (BGP) process was applied to Ha-Soo-Oh with Bacillus megaterium tyrosinase (BmTYR) as a biocatalyst. The result showed that a major biotransformed compound could be purified using the BGP process with preparative high-performance liquid chromatography (HPLC), and it was confirmed as a new compound, 2,3,5,3',4'-pentahydroxystilbene-2-O-β-glucoside (PSG) following mass and nucleic magnetic resonance (NMR) spectral analyses. PSG was further confirmed as a biotransformation product from 2,3,5,4'-tetrahydroxystilbene-2-O-β-glucoside (TSG) by BmTYR. The new PSG exhibited 4.7-fold higher 1,1-diphenyl-2-picrylhydrazine (DPPH) free radical scavenging activity than that of TSG. The present study highlights the potential usage of BGP in herbs to discover new bioactive compounds in the future.
Keywords: Ha-Soo-Oh; Polygonum multiflorum; antioxidant; biotransformation; hydroxylation; melanogenesis; tyrosinase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Tetrahydroxystilbene glucoside isolated from Polygonum multiflorum Thunb. demonstrates osteoblast differentiation promoting activity.Exp Ther Med. 2017 Oct;14(4):2845-2852. doi: 10.3892/etm.2017.4915. Epub 2017 Aug 9. Exp Ther Med. 2017. PMID: 28966672 Free PMC article.
-
Novel Ganoderma triterpenoid saponins from the biotransformation-guided purification of a commercial Ganoderma extract.J Biosci Bioeng. 2023 May;135(5):402-410. doi: 10.1016/j.jbiosc.2023.02.004. Epub 2023 Mar 6. J Biosci Bioeng. 2023. PMID: 36889998
-
Preparative isolation and purification of 12 main antioxidants from the roots of Polygonum multiflorum Thunb. using high-speed countercurrent chromatography and preparative HPLC guided by 1,1'-diphenyl-2-picrylhydrazyl-HPLC.J Sep Sci. 2020 Apr;43(8):1415-1422. doi: 10.1002/jssc.201901287. Epub 2020 Mar 5. J Sep Sci. 2020. PMID: 32003117
-
A review on the extraction, purification, detection, and pharmacological effects of 2,3,5,4'-tetrahydroxystilbene-2-O-β-d-glucoside from Polygonum multiflorum.Biomed Pharmacother. 2020 Apr;124:109923. doi: 10.1016/j.biopha.2020.109923. Epub 2020 Jan 24. Biomed Pharmacother. 2020. PMID: 31986418 Review.
-
[Recent advances in anti-aging study of 2,3,5,4'-tetrahydroxystilbene-2-O-beta-D-glucopyranoside--a main component of Polygonum multiflorum].Zhongguo Zhong Yao Za Zhi. 2016 Jan;41(2):182-185. doi: 10.4268/cjcmm20160205. Zhongguo Zhong Yao Za Zhi. 2016. PMID: 28861960 Review. Chinese.
Cited by
-
Functional Approaches to Discover New Compounds via Enzymatic Modification: Predicted Data Mining Approach and Biotransformation-Guided Purification.Molecules. 2025 May 20;30(10):2228. doi: 10.3390/molecules30102228. Molecules. 2025. PMID: 40430400 Free PMC article. Review.
-
Development of a New Isoxsuprine Hydrochloride-Based Hydroxylated Compound with Potent Antioxidant and Anti-Inflammatory Activities.J Microbiol Biotechnol. 2024 Dec 28;34(12):2693-2701. doi: 10.4014/jmb.2405.05031. Epub 2024 Oct 3. J Microbiol Biotechnol. 2024. PMID: 39467698 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

