Nutritional Niches of Cancer Therapy-Induced Senescent Cells
- PMID: 36079891
- PMCID: PMC9460569
- DOI: 10.3390/nu14173636
Nutritional Niches of Cancer Therapy-Induced Senescent Cells
Abstract
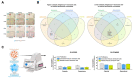
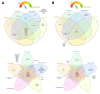
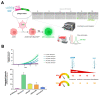
Therapy-induced senescence (TIS) is a state of stable proliferative arrest of both normal and neoplastic cells that is triggered by exposure to anticancer treatments. TIS cells acquire a senescence-associated secretory phenotype (SASP), which is pro-inflammatory and actively promotes tumor relapse and adverse side-effects in patients. Here, we hypothesized that TIS cells adapt their scavenging and catabolic ability to overcome the nutritional constraints in their microenvironmental niches. We used a panel of mechanistically-diverse TIS triggers (i.e., bleomycin, doxorubicin, alisertib, and palbociclib) and Biolog Phenotype MicroArrays to identify (among 190 different carbon and nitrogen sources) candidate metabolites that support the survival of TIS cells in limiting nutrient conditions. We provide evidence of distinguishable TIS-associated nutrient consumption profiles involving a core set of shared (e.g., glutamine) and unique (e.g., glucose-1-phosphate, inosine, and uridine) nutritional sources after diverse senescence-inducing interventions. We also observed a trend for an inverse correlation between the intensity of the pro-inflammatory SASP provoked by different TIS agents and diversity of compensatory nutritional niches utilizable by senescent cells. These findings support the detailed exploration of the nutritional niche as a new metabolic dimension to understand and target TIS in cancer.
Keywords: cancer; glutamine; metabolism; miR146a; nutrition; senescence.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022;12:31–46. doi: 10.1158/2159-8290.CD-21-1059. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

