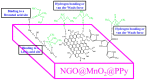
Nano-Sheet-like Morphology of Nitrogen-Doped Graphene-Oxide-Grafted Manganese Oxide and Polypyrrole Composite for Chemical Warfare Agent Simulant Detection
- PMID: 36080003
- PMCID: PMC9457797
- DOI: 10.3390/nano12172965
Nano-Sheet-like Morphology of Nitrogen-Doped Graphene-Oxide-Grafted Manganese Oxide and Polypyrrole Composite for Chemical Warfare Agent Simulant Detection
Abstract
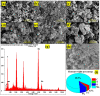
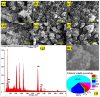
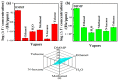
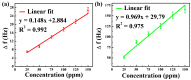
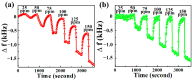
Chemical warfare agents (CWAs) have inflicted monumental damage to human lives from World War I to modern warfare in the form of armed conflict, terrorist attacks, and civil wars. Is it possible to detect the CWAs early and prevent the loss of human lives? To answer this research question, we synthesized hybrid composite materials to sense CWAs using hydrothermal and thermal reduction processes. The synthesized hybrid composite materials were evaluated with quartz crystal microbalance (QCM) and surface acoustic wave (SAW) sensors as detectors. The main findings from this study are: (1) For a low dimethyl methyl phosphonate (DMMP) concentration of 25 ppm, manganese dioxide nitrogen-doped graphene oxide (NGO@MnO2) and NGO@MnO2/Polypyrrole (PPy) showed the sensitivities of 7 and 51 Hz for the QCM sensor and 146 and 98 Hz for the SAW sensor. (2) NGO@MnO2 and NGO@MnO2/PPy showed sensitivities of more than 50-fold in the QCM sensor and 100-fold in the SAW sensor between DMMP and potential interferences. (3) NGO@MnO2 and NGO@MnO2/PPy showed coefficients of determination (R2) of 0.992 and 0.975 for the QCM sensor and 0.979 and 0.989 for the SAW sensor. (4) NGO@MnO2 and NGO@MnO2/PPy showed repeatability of 7.00 ± 0.55 and 47.29 ± 2.69 Hz in the QCM sensor and 656.37 ± 73.96 and 665.83 ± 77.50 Hz in the SAW sensor. Based on these unique findings, we propose NGO@MnO2 and NGO@MnO2/PPy as potential candidate materials that could be used to detect CWAs.
Keywords: chemical warfare agents (CWAs); dimethyl methyl phosphonate (DMMP); quartz crystal microbalance (QCM); surface acoustic wave (SAW); volatile compounds (VOCs).
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Kuča K., Pohanka M. Molecular, Clinical and Environmental Toxicology. Birkhäuser; Basel, Switzerland: 2010. Chemical warfare agents; pp. 543–558.
-
- Tu A.T. Basic information on nerve gas and the use of sarin by Aum Shinrikyo. J. Mass Spectrom. Soc. Jpn. 1996;44:293–320. doi: 10.5702/massspec.44.293. - DOI
-
- Sellström A., Cairns S., Barbeschi M. United Nations Mission to Investigate Allegations of the Use of Chemical Weapons in the Syrian Arab Republic—Final Report. United Nations Office for the Coordination of Humanitarian Affairs (OCHA); New York, NY, USA: 2013. p. 12.
-
- Gunzer F., Baether W., Zimmermann S. Investigation of dimethyl methylphosphonate (DMMP) with an Ion mobility spectrometer using a pulsed electron source. Int. J. Ion Mobil. Spectrom. 2011;14:99–107. doi: 10.1007/s12127-011-0065-x. - DOI
-
- Brunol E., Berger F., Fromm M., Planade R. Detection of dimethyl methylphosphonate (DMMP) by tin dioxide-based gas sensor: Response curve and understanding of the reactional mechanism. Sens. Actuators B Chem. 2006;120:35–41. doi: 10.1016/j.snb.2006.01.040. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

