Iron-Based Hollow Nanoplatforms for Cancer Imaging and Theranostics
- PMID: 36080059
- PMCID: PMC9457987
- DOI: 10.3390/nano12173023
Iron-Based Hollow Nanoplatforms for Cancer Imaging and Theranostics
Abstract
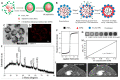
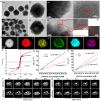
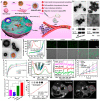
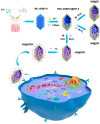
Over the past decade, iron (Fe)-based hollow nanoplatforms (Fe-HNPs) have attracted increasing attention for cancer theranostics, due to their high safety and superior diagnostic/therapeutic features. Specifically, Fe-involved components can serve as magnetic resonance imaging (MRI) contrast agents (CAs) and Fenton-like/photothermal/magnetic hyperthermia (MTH) therapy agents, while the cavities are able to load various small molecules (e.g., fluorescent dyes, chemotherapeutic drugs, photosensitizers, etc.) to allow multifunctional all-in-one theranostics. In this review, the recent advances of Fe-HNPs for cancer imaging and treatment are summarized. Firstly, the use of Fe-HNPs in single T1-weighted MRI and T2-weighted MRI, T1-/T2-weighted dual-modal MRI as well as other dual-modal imaging modalities are presented. Secondly, diverse Fe-HNPs, including hollow iron oxide (IO) nanoparticles (NPs), hollow matrix-supported IO NPs, hollow Fe-complex NPs and hollow Prussian blue (PB) NPs are described for MRI-guided therapies. Lastly, the potential clinical obstacles and implications for future research of these hollow Fe-based nanotheranostics are discussed.
Keywords: iron-based hollow nanoplatforms; magnetic resonance imaging; multifunctional; theranostics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Liao G., He F., Li Q., Zhong L., Zhao R., Che H., Gao H., Fang B. Emerging graphitic carbon nitride-based materials for biomedical applications. Prog. Mater Sci. 2020;112:100666. doi: 10.1016/j.pmatsci.2020.100666. - DOI
-
- Pallares R.M., Abergel R.J. Nanoparticles for targeted cancer radiotherapy. Nano Res. 2020;13:2887–2897. doi: 10.1007/s12274-020-2957-8. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

