Radionuclides for Targeted Therapy: Physical Properties
- PMID: 36080198
- PMCID: PMC9457625
- DOI: 10.3390/molecules27175429
Radionuclides for Targeted Therapy: Physical Properties
Abstract
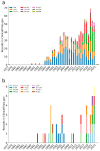
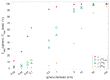
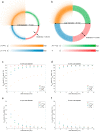
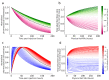
A search in PubMed revealed that 72 radionuclides have been considered for molecular or functional targeted radionuclide therapy. As radionuclide therapies increase in number and variations, it is important to understand the role of the radionuclide and the various characteristics that can render it either useful or useless. This review focuses on the physical characteristics of radionuclides that are relevant for radionuclide therapy, such as linear energy transfer, relative biological effectiveness, range, half-life, imaging properties, and radiation protection considerations. All these properties vary considerably between radionuclides and can be optimised for specific targets. Properties that are advantageous for some applications can sometimes be drawbacks for others; for instance, radionuclides that enable easy imaging can introduce more radiation protection concerns than others. Similarly, a long radiation range is beneficial in targets with heterogeneous uptake, but it also increases the radiation dose to tissues surrounding the target, and, hence, a shorter range is likely more beneficial with homogeneous uptake. While one cannot select a collection of characteristics as each radionuclide comes with an unchangeable set, all the 72 radionuclides investigated for therapy-and many more that have not yet been investigated-provide numerous sets to choose between.
Keywords: alpha; auger; beta; molecular radiotherapy; radioactivity; radionuclide; radionuclide therapy; targeted therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

