Small-Molecule PROTACs for Cancer Immunotherapy
- PMID: 36080223
- PMCID: PMC9458232
- DOI: 10.3390/molecules27175439
Small-Molecule PROTACs for Cancer Immunotherapy
Abstract
Unsatisfactory physicochemical properties of macromolecular drugs seriously hinder their application in tumor immunotherapy. However, these problems can be effectively solved by small-molecule compounds. In the promising field of small-molecule drug development, proteolysis targeting chimera (PROTAC) offers a novel mode of action in the interactions between small molecules and therapeutic targets (mainly proteins). This revolutionary technology has shown considerable impact on several proteins related to tumor survival but is rarely exploited in proteins associated with immuno-oncology up until now. This review attempts to comprehensively summarize the well-studied and less-developed immunological targets available for PROTAC technology, as well as some targets to be explored, aiming to provide more options and opportunities for the development of small-molecule-based tumor immunotherapy. In addition, some novel directions that can magnify and broaden the protein degradation efficiency are mentioned to improve PROTAC design in the future.
Keywords: proteolysis targeting chimera; small molecule inhibitors; targeted protein degradation; tumor immunotherapy; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

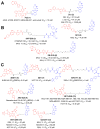

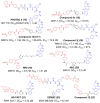




Similar articles
-
Proteolysis-Targeting Chimeras (PROTACs) in Cancer Therapy: Present and Future.Molecules. 2022 Dec 12;27(24):8828. doi: 10.3390/molecules27248828. Molecules. 2022. PMID: 36557960 Free PMC article. Review.
-
Novel strategies and promising opportunities for targeted protein degradation: An innovative therapeutic approach to overcome cancer resistance.Pharmacol Ther. 2023 Apr;244:108371. doi: 10.1016/j.pharmthera.2023.108371. Epub 2023 Mar 5. Pharmacol Ther. 2023. PMID: 36871783 Review.
-
Targeting micro-environmental pathways by PROTACs as a therapeutic strategy.Semin Cancer Biol. 2022 Nov;86(Pt 2):269-279. doi: 10.1016/j.semcancer.2022.07.001. Epub 2022 Jul 4. Semin Cancer Biol. 2022. PMID: 35798235 Free PMC article. Review.
-
Progress in the controllability technology of PROTAC.Eur J Med Chem. 2024 Feb 5;265:116096. doi: 10.1016/j.ejmech.2023.116096. Epub 2023 Dec 27. Eur J Med Chem. 2024. PMID: 38160619 Review.
-
Proteolysis-targeting chimera (PROTAC) for targeted protein degradation and cancer therapy.J Hematol Oncol. 2020 May 13;13(1):50. doi: 10.1186/s13045-020-00885-3. J Hematol Oncol. 2020. PMID: 32404196 Free PMC article. Review.
Cited by
-
Navigating PROTACs in Cancer Therapy: Advancements, Challenges, and Future Horizons.Food Sci Nutr. 2025 Feb 1;13(2):e70011. doi: 10.1002/fsn3.70011. eCollection 2025 Feb. Food Sci Nutr. 2025. PMID: 39898116 Free PMC article. Review.
-
Antibody Drug Conjugates (ADCs): Shaping the Future of Precision Oncology.Anticancer Agents Med Chem. 2025;25(14):993-1016. doi: 10.2174/0118715206348204241128063329. Anticancer Agents Med Chem. 2025. PMID: 39901681 Review.
-
Cancer-Specific Delivery of Proteolysis-Targeting Chimeras (PROTACs) and Their Application to Cancer Immunotherapy.Pharmaceutics. 2023 Jan 26;15(2):411. doi: 10.3390/pharmaceutics15020411. Pharmaceutics. 2023. PMID: 36839734 Free PMC article. Review.
-
Therapeutic Target Identification and Drug Discovery Driven by Chemical Proteomics.Biology (Basel). 2024 Jul 23;13(8):555. doi: 10.3390/biology13080555. Biology (Basel). 2024. PMID: 39194493 Free PMC article. Review.
-
Proteolysis-Targeting Chimeras (PROTACs) in Cancer Therapy: Present and Future.Molecules. 2022 Dec 12;27(24):8828. doi: 10.3390/molecules27248828. Molecules. 2022. PMID: 36557960 Free PMC article. Review.
References
-
- Li Y., Yang L., Xu X., Li M., Zhang Y., Lin Q., Gong T., Sun X., Zhang Z., Zhang L. Multifunctional Size-Expandable Nanomedicines Enhance Tumor Accumulation and Penetration for Synergistic Chemo-Photothermal Therapy. ACS Appl. Mater. Interfaces. 2021;13:46361–46374. doi: 10.1021/acsami.1c14170. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

