Antibody-Ferrocene Conjugates as a Platform for Electro-Chemical Detection of Low-Density Lipoprotein
- PMID: 36080260
- PMCID: PMC9458124
- DOI: 10.3390/molecules27175492
Antibody-Ferrocene Conjugates as a Platform for Electro-Chemical Detection of Low-Density Lipoprotein
Abstract
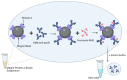
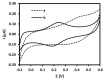
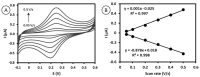
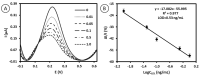
Low-density lipoprotein (LDL) is a cardiac biomarker identified in the pathology of cardiovascular disease (CVD). Typically, the level of LDL is calculated using the Friedewald relationship based on measured values of total cholesterol, high-density lipoproteins (HDL), and triglycerides. Unfortunately, this approach leads to some errors in calculation. Therefore, direct methods that can be used for fast and accurate detection of LDL are needed. The purpose of this study was to develop an electrochemical platform for the detection of LDL based on an antibody-ferrocene conjugate. An anti-apolipoprotein B-100 antibody labeled with ferrocene was covalently immobilized on the layer of 4-aminothiophenol (4-ATP) on the surface of gold electrodes. Upon interaction between LDL and the antibody-ferrocene conjugate, a decrease in the ferrocene redox signal registered by square wave voltammetry was observed, which depends linearly on the concentration from 0.01 ng/mL to 1.0 ng/mL. The obtained limit of detection was equal to 0.53 ng/mL. Moreover, the satisfied selectivity toward human serum albumin (HSA), HDL, and malondialdehyde-modified low-density lipoprotein (MDA-LDL) was observed. In addition, the acceptable recovery rates of LDL in human serum samples indicate the possible application of immunosensors presented in clinical diagnostics.
Keywords: electrochemical detection; ferrocene–antibody conjugates; immunosensor; low-density lipoprotein (LDL).
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Simultaneous Electrochemical Detection of LDL and MDA-LDL Using Antibody-Ferrocene or Anthraquinone Conjugates Coated Magnetic Beads.Int J Mol Sci. 2023 Mar 22;24(6):6005. doi: 10.3390/ijms24066005. Int J Mol Sci. 2023. PMID: 36983078 Free PMC article.
-
Detection of Low Density Lipoprotein-Comparison of Electrochemical Immuno- and Aptasensor.Sensors (Basel). 2021 Nov 20;21(22):7733. doi: 10.3390/s21227733. Sensors (Basel). 2021. PMID: 34833808 Free PMC article.
-
Comparing a novel equation for calculating low-density lipoprotein cholesterol with the Friedewald equation: A VOYAGER analysis.Clin Biochem. 2019 Feb;64:24-29. doi: 10.1016/j.clinbiochem.2018.10.011. Epub 2018 Oct 24. Clin Biochem. 2019. PMID: 30365923
-
Non-HDL-cholesterol and apolipoprotein B compared with LDL-cholesterol in atherosclerotic cardiovascular disease risk assessment.Pathology. 2019 Feb;51(2):148-154. doi: 10.1016/j.pathol.2018.11.006. Epub 2018 Dec 27. Pathology. 2019. PMID: 30595507 Review.
-
Rationale for use of non-high-density lipoprotein cholesterol rather than low-density lipoprotein cholesterol as a tool for lipoprotein cholesterol screening and assessment of risk and therapy.Am J Cardiol. 1998 Feb 26;81(4A):26B-31B. doi: 10.1016/s0002-9149(98)00034-4. Am J Cardiol. 1998. PMID: 9526810 Review.
Cited by
-
Simultaneous Electrochemical Detection of LDL and MDA-LDL Using Antibody-Ferrocene or Anthraquinone Conjugates Coated Magnetic Beads.Int J Mol Sci. 2023 Mar 22;24(6):6005. doi: 10.3390/ijms24066005. Int J Mol Sci. 2023. PMID: 36983078 Free PMC article.
-
A novel electrochemical aptasensor based on NrGO-H-Mn3O4 NPs integrated CRISPR/Cas12a system for ultrasensitive low-density lipoprotein determination.Mikrochim Acta. 2024 Aug 20;191(9):547. doi: 10.1007/s00604-024-06628-2. Mikrochim Acta. 2024. PMID: 39162876
-
The Applications of Electrochemical Immunosensors in the Detection of Disease Biomarkers: A Review.Molecules. 2023 Apr 20;28(8):3605. doi: 10.3390/molecules28083605. Molecules. 2023. PMID: 37110837 Free PMC article. Review.
-
Carbon-based light addressable potential aptasensor based on the synergy of C-MXene@rGO and OPD@NGQDs for low-density lipoprotein detection.Mikrochim Acta. 2024 Dec 27;192(1):35. doi: 10.1007/s00604-024-06909-w. Mikrochim Acta. 2024. PMID: 39729216
-
Proximity Ligation Induced DNAzyme Motor Cooperating with Exponential Amplification Reaction for Accurate Low-Density Lipoprotein (LDL) Detection.ACS Omega. 2025 Jan 27;10(4):4037-4043. doi: 10.1021/acsomega.4c10224. eCollection 2025 Feb 4. ACS Omega. 2025. PMID: 39926505 Free PMC article.
References
-
- Ference B.A., Ginsberg H.N., Graham I., Ray K.K., Packard C.J., Bruckert E., Hegele R.A., Krauss R.M., Raal F.J., Schunkert H., et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017;38:2459–2472. doi: 10.1093/eurheartj/ehx144. - DOI - PMC - PubMed
-
- Hevonoja T., Pentikäinen M.O., Hyvönen M.T., Kovanen P.T., Ala-Korpela M. Structure of low density lipoprotein (LDL) particles: Basis for understanding molecular changes in modified LDL. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids. 2000;1488:189–210. doi: 10.1016/S1388-1981(00)00123-2. - DOI - PubMed
-
- Ec M.-V., Kk R. Physiological Level of LDL Cholesterol: The Master Key a Nobel Dream Comes True. Volume 6. Walsh Medical Media; London, UK: 2016. p. 5. Cardiovascular Pharmacology.
-
- Mach F., Baigent C., Catapano A.L., Koskinas K.C., Casula M., Badimon L., Chapman M.J., De Backer G.G., Delgado V., Ference B.A., et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) Eur. Heart J. 2019;41:111–188. doi: 10.1093/eurheartj/ehz455. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

