Dual Inhibition of HIV-1 and Cathepsin L Proteases by Sarcandra glabra
- PMID: 36080318
- PMCID: PMC9457736
- DOI: 10.3390/molecules27175552
Dual Inhibition of HIV-1 and Cathepsin L Proteases by Sarcandra glabra
Abstract
The COVID-19 pandemic continues to impose a huge threat on human health due to rapid viral mutations. Thus, it is imperative to develop more potent antivirals with both prophylactic and treatment functions. In this study, we screened for potential antiviral compounds from Sarcandra glabra (SG) against Cathepsin L and HIV-1 proteases. A FRET assay was applied to investigate the inhibitory effects and UPLC-HRMS was employed to identify and quantify the bioactive components. Furthermore, molecular docking was carried out to get a glimpse of the binding of active compounds to the proteases. Our results showed that the SG extracts (SGW, SG30, SG60, and SG85) inhibited HIV-1 protease with an IC50 of 0.003~0.07 mg/mL and Cathepsin L protease with an IC50 of 0.11~0.26 mg/mL. Fourteen compounds were identified along with eight quantified from the SG extracts. Chlorogenic acid, which presented in high content in the extracts (12.7~15.76 µg/mg), possessed the most potent inhibitory activity against HIV-1 protease (IC50 = 0.026 mg/mL) and Cathepsin L protease (inhibition: 40.8% at 0.01 mg/mL). Thus, SG extracts and the active ingredients could potentially be used to prevent/treat viral infections, including SARS-CoV-2, due to their dual-inhibition functions against viral proteases.
Keywords: Cathepsin L protease; HIV-1 protease; Sarcandra glabra; UPLC-HRMS; inhibition; molecular docking.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

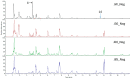

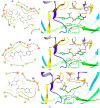
References
-
- WHO Coronavirus Disease 2019 (COVID-19): Situation Report: COVID-19 Partners Platform. [(accessed on 20 July 2022)]; Available online: https://covid19.who.int/
-
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N., Nitsche A., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. - DOI - PMC - PubMed
-
- Wei Y., Ma C.M., Li S.M., Zhou Y., Pan B.W., Xiao J.W., Yang X. Application of the Bioactivity Integrents from Du Guo Lu Huang as SARS-Cov-19 Drug. Patent Application No. 202111142665.2 (28/09/2021)
MeSH terms
Substances
Grants and funding
- Qian Ke He Zi Cheng (2020)4Y213/Guizhou Provincial Science and Technology Projects
- ZK [2022] general 480/Guizhou Provincial Science and Technology Projects
- Qian Jiao He KY (2021)018/Innovation Group Project of Guizhou Province
- 3411-4110000520364/Research Center of Molecular Bioactivity from Traditional Chinese Medicine and Ethnomedicine
- SZXK059/Shenzhen Fund for Guangdong Provincial High-level Clinical Key Specialties
- gzwkj2022-232/the projects of Guizhou province
- gzwkj2022-467/the projects of Guizhou province
- Qian Jiao He KY Zi [2022]253/the projects of Guizhou province
- 2018YFC170810204/the projects of Guizhou University of Traditional Chinese Medicine
- 2018YFC170810108/the projects of Guizhou University of Traditional Chinese Medicine
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

