Newly Designed Quinazolinone Derivatives as Novel Tyrosinase Inhibitor: Synthesis, Inhibitory Activity, and Mechanism
- PMID: 36080324
- PMCID: PMC9457556
- DOI: 10.3390/molecules27175558
Newly Designed Quinazolinone Derivatives as Novel Tyrosinase Inhibitor: Synthesis, Inhibitory Activity, and Mechanism
Abstract
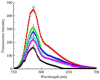
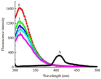
We synthesized a series of quinazolinone derivates as tyrosinase inhibitors and evaluated their inhibition constants. We synthesized 2-(2,6-dimethylhepta-1,5-dien-1-yl)quinazolin-4(3H)-one (Q1) from the natural citral. The concentration, which led to 50% activity loss of Q1, was 103 ± 2 μM (IC50 = 103 ± 2 μM). Furthermore, we considered Q1 to be a mixed-type and reversible tyrosinase inhibitor, and determined the KI and KIS inhibition constants to be 117.07 μM and 423.63 μM, respectively. Our fluorescence experiment revealed that Q1 could interact with the substrates of tyrosine and L-DOPA in addition to tyrosinase. Molecular docking studies showed that the binding of Q1 to tyrosinase was driven by hydrogen bonding and hydrophobicity. Briefly, the current study confirmed a new tyrosinase inhibitor, which is expected to be developed into a novel pigmentation drug.
Keywords: citral; derivatives; fluorescence quenching; inhibitor; molecular docking; tyrosinase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Synthesis, biological evaluations, and in silico assessments of phenylamino quinazolinones as tyrosinase inhibitors.Sci Rep. 2025 Jan 4;15(1):846. doi: 10.1038/s41598-024-81328-8. Sci Rep. 2025. PMID: 39755701 Free PMC article.
-
2-(4-Fluorophenyl)-quinazolin-4(3H)-one as a novel tyrosinase inhibitor: Synthesis, inhibitory activity, and mechanism.Bioorg Med Chem. 2016 Oct 1;24(19):4620-4625. doi: 10.1016/j.bmc.2016.07.068. Epub 2016 Aug 1. Bioorg Med Chem. 2016. PMID: 27527415
-
Evaluation of inhibitory effects of some novel phenolic derivatives on the mushroom tyrosinase activity: Insights from spectroscopic analyses, molecular docking and in vitro assays.Food Chem. 2022 Sep 1;387:132938. doi: 10.1016/j.foodchem.2022.132938. Epub 2022 Apr 9. Food Chem. 2022. PMID: 35429937
-
Design, synthesis, kinetic mechanism and molecular docking studies of novel 1-pentanoyl-3-arylthioureas as inhibitors of mushroom tyrosinase and free radical scavengers.Eur J Med Chem. 2017 Dec 1;141:273-281. doi: 10.1016/j.ejmech.2017.09.059. Epub 2017 Sep 29. Eur J Med Chem. 2017. PMID: 29040952
-
Structure and inhibition mechanism of some synthetic compounds and phenolic derivatives as tyrosinase inhibitors: review and new insight.J Biomol Struct Dyn. 2023 Jul;41(10):4798-4810. doi: 10.1080/07391102.2022.2069157. Epub 2022 May 5. J Biomol Struct Dyn. 2023. PMID: 35510568 Review.
Cited by
-
Heterocyclic Compounds as Synthetic Tyrosinase Inhibitors: Recent Advances.Int J Mol Sci. 2023 May 22;24(10):9097. doi: 10.3390/ijms24109097. Int J Mol Sci. 2023. PMID: 37240442 Free PMC article. Review.
-
Development of mercapto-phenyl-1,2,4-triazole bearing thio-quinoline as a novel class of tyrosinase inhibitors: an in vitro and in silico study.Sci Rep. 2025 Jul 14;15(1):25382. doi: 10.1038/s41598-025-09072-1. Sci Rep. 2025. PMID: 40659715 Free PMC article.
-
Synthesis and tyrosinase inhibitory activities of novel isopropylquinazolinones.BMC Chem. 2023 Jun 23;17(1):65. doi: 10.1186/s13065-023-00978-3. BMC Chem. 2023. PMID: 37353836 Free PMC article.
-
Catalytic acidic deep eutectic mixture for efficient and promising synthesis of quinazolinone and quinoxaline derivatives.RSC Adv. 2025 Jul 21;15(32):25971-25984. doi: 10.1039/d5ra03346b. eCollection 2025 Jul 21. RSC Adv. 2025. PMID: 40697466 Free PMC article.
-
Synthesis, biological evaluations, and in silico assessments of phenylamino quinazolinones as tyrosinase inhibitors.Sci Rep. 2025 Jan 4;15(1):846. doi: 10.1038/s41598-024-81328-8. Sci Rep. 2025. PMID: 39755701 Free PMC article.
References
-
- Nunes C.S., Vogel K. General perspectives of enzymes, environment preservation, and scarce natural resources—Conclusions. Enzym. Hum. Anim. Nutr. 2018:515–526. doi: 10.1016/B978-0-12-805419-2.00027-7. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

