Chemical Synthesis and Biological Activities of Amaryllidaceae Alkaloid Norbelladine Derivatives and Precursors
- PMID: 36080382
- PMCID: PMC9457815
- DOI: 10.3390/molecules27175621
Chemical Synthesis and Biological Activities of Amaryllidaceae Alkaloid Norbelladine Derivatives and Precursors
Abstract
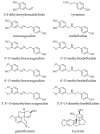
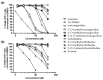
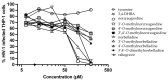
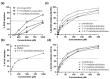
Amaryllidaceae alkaloids (AAs) are a structurally diverse family of alkaloids recognized for their many therapeutic properties, such as antiviral, anti-cholinesterase, and anticancer properties. Norbelladine and its derivatives, whose biological properties are poorly studied, are key intermediates required for the biosynthesis of all ~650 reported AAs. To gain insight into their therapeutic potential, we synthesized a series of O-methylated norbelladine-type alkaloids and evaluated their cytotoxic effects on two types of cancer cell lines, their antiviral effects against the dengue virus (DENV) and the human immunodeficiency virus 1 (HIV-1), and their anti-Alzheimer’s disease (anti-cholinesterase and -prolyl oligopeptidase) properties. In monocytic leukemia cells, norcraugsodine was highly cytotoxic (CC50 = 27.0 μM), while norbelladine was the most cytotoxic to hepatocarcinoma cells (CC50 = 72.6 μM). HIV-1 infection was impaired only at cytotoxic concentrations of the compounds. The 3,4-dihydroxybenzaldehyde (selectivity index (SI) = 7.2), 3′,4′-O-dimethylnorbelladine (SI = 4.8), 4′-O-methylnorbelladine (SI > 4.9), 3′-O-methylnorbelladine (SI > 4.5), and norcraugsodine (SI = 3.2) reduced the number of DENV-infected cells with EC50 values ranging from 24.1 to 44.9 μM. The O-methylation of norcraugsodine abolished its anti-DENV potential. Norbelladine and its O-methylated forms also displayed butyrylcholinesterase-inhibition properties (IC50 values ranging from 26.1 to 91.6 μM). Altogether, the results provided hints of the structure−activity relationship of norbelladine-type alkaloids, which is important knowledge for the development of new inhibitors of DENV and butyrylcholinesterase.
Keywords: Alzheimer’s disease; Amaryllidaceae alkaloid; O-methylation; anti-cholinesterase; antiviral; dengue virus; galanthamine; norbelladine; specialized metabolism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

