Synthesis of thia-Michael-Type Adducts between Naphthoquinones and N-Acetyl- L-Cysteine and Their Biological Activity
- PMID: 36080409
- PMCID: PMC9457610
- DOI: 10.3390/molecules27175645
Synthesis of thia-Michael-Type Adducts between Naphthoquinones and N-Acetyl- L-Cysteine and Their Biological Activity
Abstract
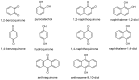
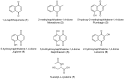
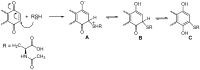
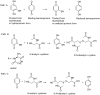
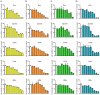
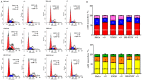
A series of naphthoquinones, namely, 1,4-naphthoquinone, menadione, plumbagin, juglone, naphthazarin, and lawsone, were reacted with N-acetyl-L-cysteine, and except for lawsone, which did not react, the related adducts were obtained. After the tuning of the solvent and reaction conditions, the reaction products were isolated as almost pure from the complex reaction mixture via simple filtration and were fully characterized. Therefore, the aim of this work was to evaluate whether the antitumor activity of new compounds of 1,4-naphthoquinone derivatives leads to an increase in ROS in tumor cell lines of cervical carcinoma (HeLa), neuroblastoma (SH-SY5Y), and osteosarcoma (SaOS2, U2OS) and in normal dermal fibroblast (HDFa). The MTT assay was used to assay cell viability, the DCF-DA fluorescent probe to evaluate ROS induction, and cell-cycle analysis to measure the antiproliferative effect. Compounds 8, 9, and 12 showed a certain degree of cytotoxicity towards all the malignant cell lines tested, while compound 11 showed biological activity at higher IC50 values. Compounds 8 and 11 induced increases in ROS generation after 1 h of exposure, while after 48 h of treatment, only 8 induced an increase in ROS formation in HeLa cells. Cell-cycle analysis showed that compound 8 caused an increase in the number of G0/G1-phase cells in the HeLa experiment, while for the U2OS and SH-SY5Y cell lines, it led to an accumulation of S-phase cells. Therefore, these novel 1,4-naphthoquinone derivatives may be useful as antitumoral agents in the treatment of different cancers.
Keywords: N-acetyl-L-cysteine; cancer; juglone; menadione; naphthazarin; naphthoquinone; plumbagin; thia-Michael.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

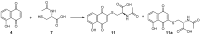
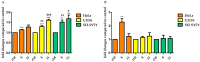
References
-
- Patai S., Rappoport Z., editors. The Quinonoid Compounds: Part 1. Volume 1. John Wiley & Sons Ltd.; New York, NY, USA: 1988. - DOI
-
- Patai S., Rappoport Z., editors. The Quinonoid Compounds: Part 2. Volume 2. John Wiley & Sons Ltd.; New York, NY, USA: 1988. - DOI
-
- Dulo B., Phan K., Githaiga J., Raes K., De Meester S. Natural Quinone Dyes: A Review on Structure, Extraction Techniques, Analysis and Application Potential. Waste Biomass-Valorization. 2021;12:6339–6374. doi: 10.1007/s12649-021-01443-9. - DOI
-
- Boga C., Delpivo C., Ballarin B., Morigi M., Galli S., Micheletti G., Tozzi S. Investigation on the dyeing power of some organic natural compounds for a green approach to hair dyeing. Dyes Pigments. 2013;97:9–18. doi: 10.1016/j.dyepig.2012.11.020. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

