Scalable (Enantioselective) Syntheses of Novel 3-Methylated Analogs of Pazinaclone, (S)-PD172938 and Related Biologically Relevant Isoindolinones
- PMID: 36080411
- PMCID: PMC9458024
- DOI: 10.3390/molecules27175647
Scalable (Enantioselective) Syntheses of Novel 3-Methylated Analogs of Pazinaclone, (S)-PD172938 and Related Biologically Relevant Isoindolinones
Abstract
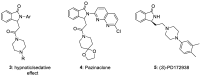
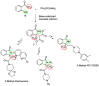
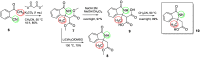
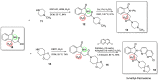
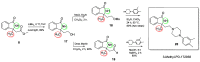
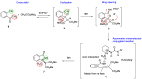
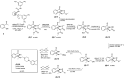
Herein, we report the application of an efficient and practical K2CO3 promoted cascade reaction of 2-acetylbenzonitrile in the synthesis of novel 3-methylated analogs of Pazinaclone and PD172938, belonging to isoindolinones heterocyclic class bearing a tetrasubstituted stereocenter. Organocatalytic asymmetric synthesis of the key intermediate and its transformation into highly enantioenriched 3-methylated analog of (S)-PD172938 was also developed. These achievements can be of particular interest also for medicinal chemistry, since the methyl group is a very useful structural modification in the rational design of new and more effective bioactive compounds.
Keywords: asymmetric synthesis; heterocycles; isoindolinones.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
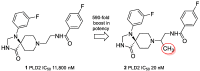
Similar articles
-
Isoindolinones as Michael Donors under Phase Transfer Catalysis: Enantioselective Synthesis of Phthalimidines Containing a Tetrasubstituted Carbon Stereocenter.Molecules. 2015 May 12;20(5):8484-98. doi: 10.3390/molecules20058484. Molecules. 2015. PMID: 25985353 Free PMC article.
-
Enantioselective pharmacokinetics in animals of pazinaclone, a new isoindoline anxiolytic, and its active metabolite.Biopharm Drug Dispos. 1995 Dec;16(9):755-73. doi: 10.1002/bdd.2510160906. Biopharm Drug Dispos. 1995. PMID: 8580400
-
Cobalt-Catalyzed Enantioselective C-H Carbonylation towards Chiral Isoindolinones.Angew Chem Int Ed Engl. 2024 Mar 4;63(10):e202318803. doi: 10.1002/anie.202318803. Epub 2024 Jan 24. Angew Chem Int Ed Engl. 2024. PMID: 38205884
-
From Three- to Six-Membered Heterocycles Bearing a Quaternary Stereocenter: an Asymmetric Organocatalytic Approach.Chem Rec. 2023 May;23(5):e202300066. doi: 10.1002/tcr.202300066. Epub 2023 Apr 12. Chem Rec. 2023. PMID: 37042434 Review.
-
Catalytic asymmetric synthesis of enantioenriched heterocycles bearing a C-CF3 stereogenic center.Chemistry. 2015 Jun 8;21(24):8664-84. doi: 10.1002/chem.201500361. Epub 2015 Mar 3. Chemistry. 2015. PMID: 25736019 Review.
Cited by
-
Asymmetric Organocatalytic Mannich Reaction in the Synthesis of Hybrid Isoindolinone-Pyrazole and Isoindolinone-Aminal from Functionalized α-Amidosulfone.Int J Mol Sci. 2023 Mar 17;24(6):5783. doi: 10.3390/ijms24065783. Int J Mol Sci. 2023. PMID: 36982854 Free PMC article.
-
Catalyst-Free Spontaneous Aza-Mannich/Lactamization Cascade Reaction: Easy Access to Polycyclic δ-Lactams.Molecules. 2025 Jun 23;30(13):2702. doi: 10.3390/molecules30132702. Molecules. 2025. PMID: 40649220 Free PMC article.
References
-
- McGrath N.A., Brichacek M., Njardarson J.T. A Graphical Journey of Innovative Organic Architectures That Have Improved Our Lives. J. Chem. Educ. 2010;87:1348–1349. doi: 10.1021/ed1003806. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

