Structure-Activity Relationship Development Efforts towards Peripherally Selective Analogs of the Cannabinoid Receptor Partial Agonist BAY 59-3074
- PMID: 36080443
- PMCID: PMC9457575
- DOI: 10.3390/molecules27175672
Structure-Activity Relationship Development Efforts towards Peripherally Selective Analogs of the Cannabinoid Receptor Partial Agonist BAY 59-3074
Abstract
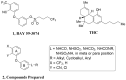
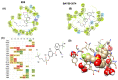
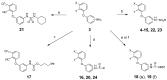
Selective modulation of peripheral cannabinoid receptors (CBRs) has potential therapeutic applications in medical conditions, including obesity, diabetes, liver diseases, GI disorders and pain. While there have been considerable efforts to produce selective antagonists or full agonists of CBRs, there has been limited reports on the development of partial agonists. Partial agonists targeting peripheral CBRs may have desirable pharmacological profiles while not producing centrally mediated dissociative effects. Bayer reported that BAY 59-3074 is a CNS penetrant partial agonist of both CB1 and CB2 receptors with efficacy in rat models of neuropathic and inflammatory pain. In this report, we demonstrate our efforts to synthesize analogs that would favor peripheral selectivity, while maintaining partial agonism of CB1. Our efforts led to the identification of a novel compound, which is a partial agonist of the human CB1 (hCB1) receptor with vastly diminished brain exposure compared to BAY 59-3074.
Keywords: CB1; CB2; agonist; cannabinoid; ligand; partial; peripheral.
Conflict of interest statement
The authors do not have known conflict of interest.
Figures
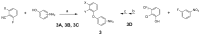
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

