Exploring The Relative Astringency of Tea Catechins and Distinct Astringent Sensation of Catechins and Flavonol Glycosides via an In Vitro Assay Composed of Artificial Oil Bodies
- PMID: 36080445
- PMCID: PMC9457659
- DOI: 10.3390/molecules27175679
Exploring The Relative Astringency of Tea Catechins and Distinct Astringent Sensation of Catechins and Flavonol Glycosides via an In Vitro Assay Composed of Artificial Oil Bodies
Abstract
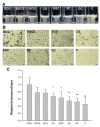
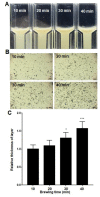
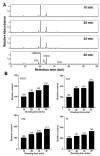
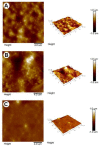
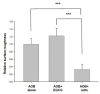
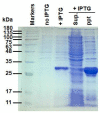
Artificial oil bodies covered by a recombinant surface protein, caleosin fused with histatin 3 (a major human salivary peptide), were employed to explore the relative astringency of eight tea catechins. The results showed that gallate-type catechins were more astringent than non-gallate-type catechins, with an astringency order of epicatechin gallate > epigallocatechin gallate > gallocatechin gallate > catechin gallate > epigallocatechin > epicatechin > gallocatechin > catechin. As expected, the extension of brewing time led to an increase in catechin content in the tea infusion, thus elevating tea astringency. Detailed analysis showed that the enhanced proportion of gallate-type catechins was significantly higher than that of non-gallate-type catechins, indicating that tea astringency was elevated exponentially, rather than proportionally, when brewing time was extended. Rough surfaces were observed on artificial oil bodies when they were complexed with epigallocatechin gallate (a catechin), while a smooth surface was observed on those complexed with rutin (a flavonol glycoside) under an atomic force microscope and a scanning electron microscope. The results indicate that catechins and flavonol glycosides induce the sensation of rough (puckering) and smooth (velvety) astringency in tea, respectively.
Keywords: artificial oil bodies; astringency; caleosin; catechins; histatin 3.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Shi J., Yang G., You Q., Sun S., Chen R., Lin Z., Simal-Gandara J., Lv H. Updates on the chemistry, processing characteristics, and utilization of tea flavonoids in last two decades (2001–2021) Crit. Rev. Food Sci. Nutr. 2021;13:1–28. - PubMed
-
- Hara Y. Elucidation of physiological functions of tea catechins and their practical applications. J. Drug Food Anal. 2012;20:296–300. doi: 10.38212/2224-6614.2096. - DOI
-
- Wang S., Zeng T., Zhao S., Zhu Y., Feng C., Zhan J., Li S., Ho C.T., Gosslau A. Multifunctional health-promoting effects of oolong tea and its products. Food Sci. Hum. Well. 2022;11:512–523. doi: 10.1016/j.fshw.2021.12.009. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

