New 1,2,3-Triazole-Coumarin-Glycoside Hybrids and Their 1,2,4-Triazolyl Thioglycoside Analogs Targeting Mitochondria Apoptotic Pathway: Synthesis, Anticancer Activity and Docking Simulation
- PMID: 36080455
- PMCID: PMC9458111
- DOI: 10.3390/molecules27175688
New 1,2,3-Triazole-Coumarin-Glycoside Hybrids and Their 1,2,4-Triazolyl Thioglycoside Analogs Targeting Mitochondria Apoptotic Pathway: Synthesis, Anticancer Activity and Docking Simulation
Abstract
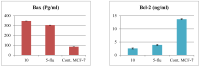
Toxicity and resistance to newly synthesized anticancer drugs represent a challenging phenomenon of intensified concern arising from variation in drug targets and consequently the prevalence of the latter concern requires further research. The current research reports the design, synthesis, and anticancer activity of new 1,2,3-triazole-coumarin-glycosyl hybrids and their 1,2,4-triazole thioglycosides as well as acyclic analogs. The cytotoxic activity of the synthesized products was studied against a panel of human cancer cell lines. Compounds 8, 10, 16 and 21 resulted in higher activities against different human cancer cells. The impact of the hybrid derivative 10 upon different apoptotic protein markers, including cytochrome c, Bcl-2, Bax, and caspase-7 along with its effect on the cell cycle was investigated. It revealed a mitochondria-apoptotic effect on MCF-7 cells and had the ability to upregulate pro-apoptotic Bax protein and downregulate anti-apoptotic Bcl-2 protein and thus implies the apoptotic fate of the cells. Furthermore, the inhibitory activities against EGFR, VEGFR-2 and CDK-2/cyclin A2 kinases for 8, 10 and 21 were studied to detect the mechanism of their high potency. The coumarin-triazole-glycosyl hybrids 8 and 10 illustrated excellent broad inhibitory activity (IC50= 0.22 ± 0.01, 0.93 ± 0.42 and 0.24 ± 0.20 μM, respectively, for compound 8), (IC50 = 0.12 ± 0.50, 0.79 ± 0.14 and 0.15± 0. 60 μM, respectively, for compound 10), in comparison with the reference drugs, erlotinib, sorafenib and roscovitine (IC50 = 0.18 ± 0.05, 1.58 ± 0.11 and 0.46 ± 0.30 μM, respectively). In addition, the docking study was simulated to afford better rationalization and put insight into the binding affinity between the promising derivatives and their targeted enzymes and that might be used as an optimum lead for further modification in the anticancer field.
Keywords: 1,2,3-triazoles; 1,2,4-triazoles; CDK-2; EGFR; anticancer; coumarin; glycosides; molecular docking.
Conflict of interest statement
The authors declare no conflict of interest.
Figures













Similar articles
-
Novel 1,2,3-Triazole-Coumarin Hybrid Glycosides and Their Tetrazolyl Analogues: Design, Anticancer Evaluation and Molecular Docking Targeting EGFR, VEGFR-2 and CDK-2.Molecules. 2022 Mar 22;27(7):2047. doi: 10.3390/molecules27072047. Molecules. 2022. PMID: 35408446 Free PMC article.
-
Novel quinazolin-4-one based derivatives bearing 1,2,3-triazole and glycoside moieties as potential cytotoxic agents through dual EGFR and VEGFR-2 inhibitory activity.Sci Rep. 2024 Oct 23;14(1):24980. doi: 10.1038/s41598-024-73171-8. Sci Rep. 2024. PMID: 39443462 Free PMC article.
-
Synthesis, anticancer effect and molecular modeling of new thiazolylpyrazolyl coumarin derivatives targeting VEGFR-2 kinase and inducing cell cycle arrest and apoptosis.Bioorg Chem. 2019 Apr;85:253-273. doi: 10.1016/j.bioorg.2018.12.040. Epub 2019 Jan 3. Bioorg Chem. 2019. PMID: 30641320
-
Targeting the interplay between MMP-2, CA II and VEGFR-2 via new sulfonamide-tethered isomeric triazole hybrids; Microwave-assisted synthesis, computational studies and evaluation.Bioorg Chem. 2022 Jul;124:105816. doi: 10.1016/j.bioorg.2022.105816. Epub 2022 Apr 16. Bioorg Chem. 2022. PMID: 35489270 Review.
-
Novel benzothiazole-based dual VEGFR-2/EGFR inhibitors targeting breast and liver cancers: Synthesis, cytotoxic activity, QSAR and molecular docking studies.Bioorg Med Chem Lett. 2022 Feb 15;58:128529. doi: 10.1016/j.bmcl.2022.128529. Epub 2022 Jan 7. Bioorg Med Chem Lett. 2022. PMID: 35007724 Review.
Cited by
-
New sulfonamide-based glycosides incorporated 1,2,3-triazole as cytotoxic agents through VEGFR-2 and carbonic anhydrase inhibitory activity.Sci Rep. 2024 Jun 6;14(1):13028. doi: 10.1038/s41598-024-62864-9. Sci Rep. 2024. PMID: 38844493 Free PMC article.
-
New thiazolidin-4-ones as anti-cervical cancer agents targeting EGFR: design, synthesis, and computational studies.Future Med Chem. 2025 Jan;17(1):75-91. doi: 10.1080/17568919.2024.2437976. Epub 2024 Dec 9. Future Med Chem. 2025. PMID: 39651653
-
New pyrazolopyridine and pyrazolothiazole-based compounds as anti-proliferative agents targeting c-Met kinase inhibition: design, synthesis, biological evaluation, and computational studies.RSC Adv. 2023 Apr 25;13(19):12889-12905. doi: 10.1039/d3ra01931d. eCollection 2023 Apr 24. RSC Adv. 2023. PMID: 37114032 Free PMC article.
-
Advances in VEGFR Inhibitors: A Comprehensive Review of Novel Anticancer Agents.Anticancer Agents Med Chem. 2025;25(10):663-687. doi: 10.2174/0118715206356712241202112641. Anticancer Agents Med Chem. 2025. PMID: 39810521 Review.
-
Coumarin as an Elite Scaffold in Anti-Breast Cancer Drug Development: Design Strategies, Mechanistic Insights, and Structure-Activity Relationships.Biomedicines. 2024 May 27;12(6):1192. doi: 10.3390/biomedicines12061192. Biomedicines. 2024. PMID: 38927399 Free PMC article. Review.
References
-
- Yao X., Cao Y., Lu L., Xu Y., Chen H., Liu C., Tao Z. Plasmodium infection suppresses colon cancer growth by inhibiting proliferation and promoting apoptosis associated with disrupting mitochondrial biogenesis and mitophagy in mice. Parasit. Vectors. 2022;15:192. doi: 10.1186/s13071-022-05291-x. - DOI - PMC - PubMed
-
- Gerber D.E. Targeted therapies: A new generation of cancer treatments. Am. Fam. Physician. 2008;77:311–319. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

