Detailed Structural Analysis of the Immunoregulatory Polysaccharides from the Mycobacterium Bovis BCG
- PMID: 36080458
- PMCID: PMC9458083
- DOI: 10.3390/molecules27175691
Detailed Structural Analysis of the Immunoregulatory Polysaccharides from the Mycobacterium Bovis BCG
Abstract
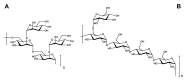
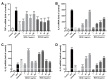
Bacillus Calmette-Guérin polysaccharide and nucleic acid (BCG-PSN), extracted from Mycobacterium bovis, is an immunoregulatory medicine commonly used in clinic. However, the structural characteristics and potential pharmacological efficacy of the polysaccharides from BCG-PSN remain unclear. Herein, two polysaccharides (BCG-1 and BCG-2) were purified and their structures were characterized. Monosaccharide composition analysis combined with methylation analysis and NMR data indicated that BCG-1 and BCG-2 were an α-D-(1→4)-mannan with (1→2)-linked branches, and an α-D-(1→4)-glucan with (1→6)-linked branches, respectively. Herein, the mannan from BCG-PSN was first reported. Bioactivity assays showed that BCG-1 and BCG-2 dose-dependently and potently increased the production of inflammatory mediators (NO, TNF-α, IL-6, IL-1β, and IL-10), as well as their mRNA expressions in RAW264.7 cells; both have similar or stronger effects compared with BCG-PSN injection. These data suggest that BCG-1 and BCG-2 are very likely the active ingredients of BCG-PSN.
Keywords: active ingredients; glucan; immunomodulator; mannan; polysaccharide purification.
Conflict of interest statement
The authors state no conflict of interest.
Figures






References
-
- Liu W., Wang H., Yu J., Liu Y., Lu W., Chai Y., Liu C., Pan C., Yao W., Gao X. Structure, chain conformation, and immunomodulatory activity of the polysaccharide purified from Bacillus Calmette Guerin formulation. Carbohydr. Polym. 2016;150:149–158. - PubMed
-
- Sharquie K.E., Hayani R.K. BCG as a new therapeutic and prophylactic agent in patients with severe oral aphthosis. Clin. Exp. Rheumatol. 2005;23:914. - PubMed
-
- Li N., Cao N., Niu Y.-D., Bai X.-H., Lu J., Sun Y., Yu M., Sun L.-X., Duan X.-S. Effects of the polysaccharide nucleic acid fraction of bacillus Calmette-Guérin on the production of interleukin-2 and interleukin-10 in the peripheral blood lymphocytes of patients with chronic idiopathic urticaria. Biomed. Rep. 2013;1:713–718. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

